Am Fam Physician. 2011;84(9):1027-1033
A more recent article on osteomyelitis is available.
Related letter: Hyperbaric Oxygen Therapy for Chronic Refractory Osteomyelitis
Patient information: See related handout on osteomyelitis, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
The incidence of chronic osteomyelitis is increasing because of the prevalence of predisposing conditions such as diabetes mellitus and peripheral vascular disease. The increased availability of sensitive imaging tests, such as magnetic resonance imaging and bone scintigraphy, has improved diagnostic accuracy and the ability to characterize the infection. Plain radiography is a useful initial investigation to identify alternative diagnoses and potential complications. Direct sampling of the wound for culture and antimicrobial sensitivity is essential to target treatment. The increased incidence of methicillin-resistant Staphylococcus aureus osteomyelitis complicates antibiotic selection. Surgical debridement is usually necessary in chronic cases. The recurrence rate remains high despite surgical intervention and long-term antibiotic therapy. Acute hematogenous osteomyelitis in children typically can be treated with a four-week course of antibiotics. In adults, the duration of antibiotic treatment for chronic osteomyelitis is typically several weeks longer. In both situations, however, empiric antibiotic coverage for S. aureus is indicated.
Osteomyelitis is generally categorized as acute or chronic based on histopathologic findings, rather than duration of the infection. Acute osteomyelitis is associated with inflammatory bone changes caused by pathogenic bacteria, and symptoms typically present within two weeks after infection. Necrotic bone is present in chronic osteomyelitis, and symptoms may not occur until six weeks after the onset of infection.1 Further classification of osteomyelitis is based on the presumed mechanism of infection (e.g., hematogenous or direct inoculation of bacteria into bone from contiguous soft tissue infection or a chronic overlying open wound).2 The more complex Cierny-Mader classification system was developed to help guide surgical management, but is generally not used in primary care.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The preferred diagnostic criterion for osteomyelitis is a positive bacterial culture from bone biopsy in the setting of bone necrosis. | C | 17, 21 |
| Magnetic resonance imaging is as sensitive as and more specific than bone scintigraphy in the diagnosis of osteomyelitis. | C | 27–30 |
| Parenteral followed by oral antibiotic therapy is as effective as long-term parenteral therapy for the treatment of chronic osteomyelitis in adults. | B | 31, 36 |
Etiology
The most common pathogens in osteomyelitis depend on the patient's age. Staphylococcus aureus is the most common cause of acute and chronic hematogenous osteomyelitis in adults and children. Group A streptococcus, Streptococcus pneumoniae, and Kingella kingae are the next most common pathogens in children. Group B streptococcal infection occurs primarily in newborns.4 In adults, S. aureus is the most common pathogen in bone and prosthetic joint infections. Increasingly, methicillin-resistant S. aureus (MRSA) is isolated from patients with osteomyelitis. In some studies, MRSA accounted for more than one-third of staphylococcal isolates.5 In more chronic cases that may be caused by contiguous infection, Staphylococcus epidermidis, Pseudomonas aeruginosa, Serratia marcescens, and Escherichia coli may be isolated. Fungal and mycobacterial infections have been reported in patients with osteomyelitis, but these are uncommon and are generally found in patients with impaired immune function.6
Clinical Features
Acute hematogenous osteomyelitis results from bacteremic seeding of bone. Children are most often affected because the metaphyseal (growing) regions of the long bones are highly vascular and susceptible to even minor trauma. More than one-half of cases of acute hematogenous osteomyelitis in children occur in patients younger than five years.7 Children typically present within two weeks of disease onset with systemic symptoms, including fever and irritability, as well as local erythema, swelling, and tenderness over the involved bone.8 Chronic osteomyelitis in children is uncommon.9
Chronic osteomyelitis is generally secondary to open fractures, bacteremia, or contiguous soft issue infection. The incidence of significant infection within three months after an open fracture has been reported to be as high as 27 percent.10 The incidence appears to be independent of the length of time from the injury to surgery.10 Only 1 to 2 percent of prosthetic joints become infected.11
Hematogenous osteomyelitis is much less common in adults than in children. It typically involves the vertebrae, but can occur in the long bones, pelvis, or clavicle. Patients with vertebral osteomyelitis often have underlying medical conditions (e.g., diabetes mellitus, cancer, chronic renal disease) or a history of intravenous drug use.12 Back pain is the primary presenting symptom.
Chronic osteomyelitis from contiguous soft tissue infection is becoming more common because of the increasing prevalence of diabetic foot infections and peripheral vascular disease. Up to one-half of patients with diabetes develop peripheral neuropathy, which may reduce their awareness of wounds and increase the risk of unrecognized infections.13 Peripheral vascular disease, which is also common in patients with diabetes, reduces the body's healing response and contributes to chronically open wounds and subsequent soft tissue infection. These conditions may act synergistically to significantly increase the risk of osteomyelitis in these patients.14
Clinical symptoms of osteomyelitis can be nonspecific and difficult to recognize. They include chronic pain, persistent sinus tract or wound drainage, poor wound healing, malaise, and sometimes fever.
Diagnosis
Acute osteomyelitis in children is primarily a clinical diagnosis based on the rapid onset and localization of symptoms. Systemic symptoms such as fever, lethargy, and irritability may be present. The physical examination should focus on identifying common findings, such as erythema, soft tissue swelling or joint effusion, decreased joint range of motion, and bony tenderness. The identification of a bacterial infection may be difficult because blood cultures are positive in only about one-half of cases.15 Because of the difficulty of diagnosis, the potential severity of infection in children, the high disease recurrence rate in adults, and the possible need for surgical intervention, consultation with an infectious disease subspecialist and an orthopedic subspecialist or plastic surgeon is advised.16
The diagnosis of osteomyelitis in adults can be difficult. A high index of clinical suspicion is required, along with recognition of clinical symptoms and supportive laboratory and imaging studies (Table 1).17 The initial evaluation should include questions to determine the patient's history of systemic symptoms (e.g., lethargy, malaise, extremity or back pain, fever) and predisposing factors (e.g., diabetes, peripheral vascular disease, history of trauma or intravenous drug use). The physical examination should focus on locating a possible nidus of infection, assessing peripheral vascular and sensory function, and exploring any ulcers for the presence of bone. If a contiguous infection with ulcer is present, such as in diabetic foot infections, the use of a sterile steel probe to detect bone may be helpful in confirming the presence of osteomyelitis. Although a 1995 study found that this test had a positive predictive value of 89 percent,18 a more recent study in a population with a lower prevalence of osteomyelitis found a positive predictive value of only 57 percent.19
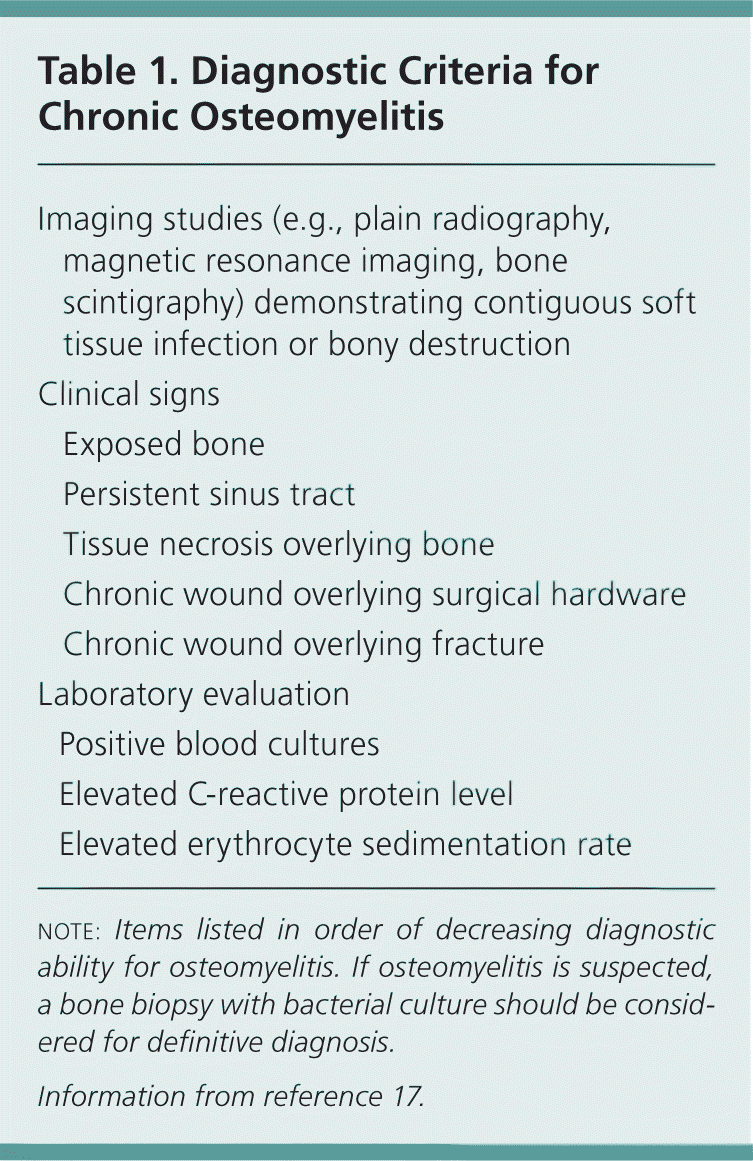
| Imaging studies (e.g., plain radiography, magnetic resonance imaging, bone scintigraphy) demonstrating contiguous soft tissue infection or bony destruction | |
| Clinical signs | |
| Exposed bone | |
| Persistent sinus tract | |
| Tissue necrosis overlying bone | |
| Chronic wound overlying surgical hardware | |
| Chronic wound overlying fracture | |
| Laboratory evaluation | |
| Positive blood cultures | |
| Elevated C-reactive protein level | |
| Elevated erythrocyte sedimentation rate | |
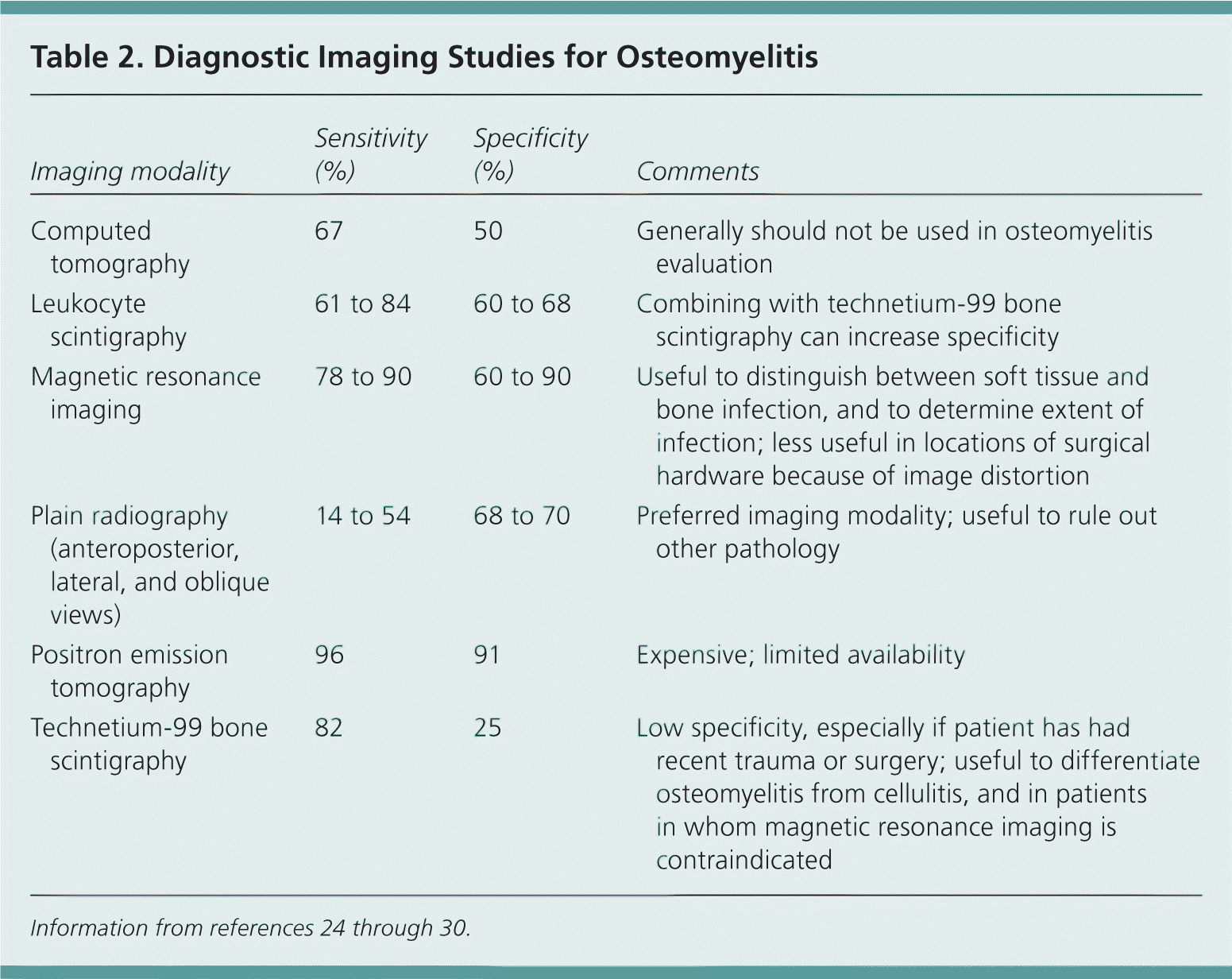
| Imaging modality | Sensitivity (%) | Specificity (%) | Comments |
|---|---|---|---|
| Computed tomography | 67 | 50 | Generally should not be used in osteomyelitis evaluation |
| Leukocyte scintigraphy | 61 to 84 | 60 to 68 | Combining with technetium-99 bone scintigraphy can increase specificity |
| Magnetic resonance imaging | 78 to 90 | 60 to 90 | Useful to distinguish between soft tissue and bone infection, and to determine extent of infection; less useful in locations of surgical hardware because of image distortion |
| Plain radiography(anteroposterior, lateral, and oblique views) | 14 to 54 | 68 to 70 | Preferred imaging modality; useful to rule out other pathology |
| Positron emission tomography | 96 | 91 | Expensive; limited availability |
| Technetium-99 bone scintigraphy | 82 | 25 | Low specificity, especially if patient has had recent trauma or surgery; useful to differentiate osteomyelitis from cellulitis, and in patients in whom magnetic resonance imaging is contraindicated |
Laboratory investigations can be helpful, but generally lack specificity for osteomyelitis. Leukocytosis and increased erythrocyte sedimentation rate and C-reactive protein levels may be present. These inflammatory markers are especially likely to be elevated in children with acute osteomyelitis. A persistently normal erythrocyte sedimentation rate and C-reactive protein level virtually rule out osteomyelitis.20 The C-reactive protein level correlates with clinical response to therapy and may be used to monitor treatment.8
Microbial cultures are essential in the diagnosis and treatment of osteomyelitis. The preferred diagnostic criteria for osteomyelitis are a positive culture from bone biopsy and histopathology consistent with necrosis.17,21 Few studies have assessed treatment outcomes based primarily on bone biopsy results. Positive blood cultures may obviate the need for a bone biopsy, especially when they are combined with substantial clinical or radiographic evidence of osteomyelitis. Superficial wound cultures do not contribute significantly to the diagnosis of osteomyelitis; the organisms identified by such cultures correspond with bone biopsy culture results in only about one-third of cases.22 Chronic infections are more likely to have polymicrobial involvement, including anaerobic, mycobacterial, and fungal organisms. Specific cultures or microbiologic testing may be required for suspected pathogens.23
IMAGING
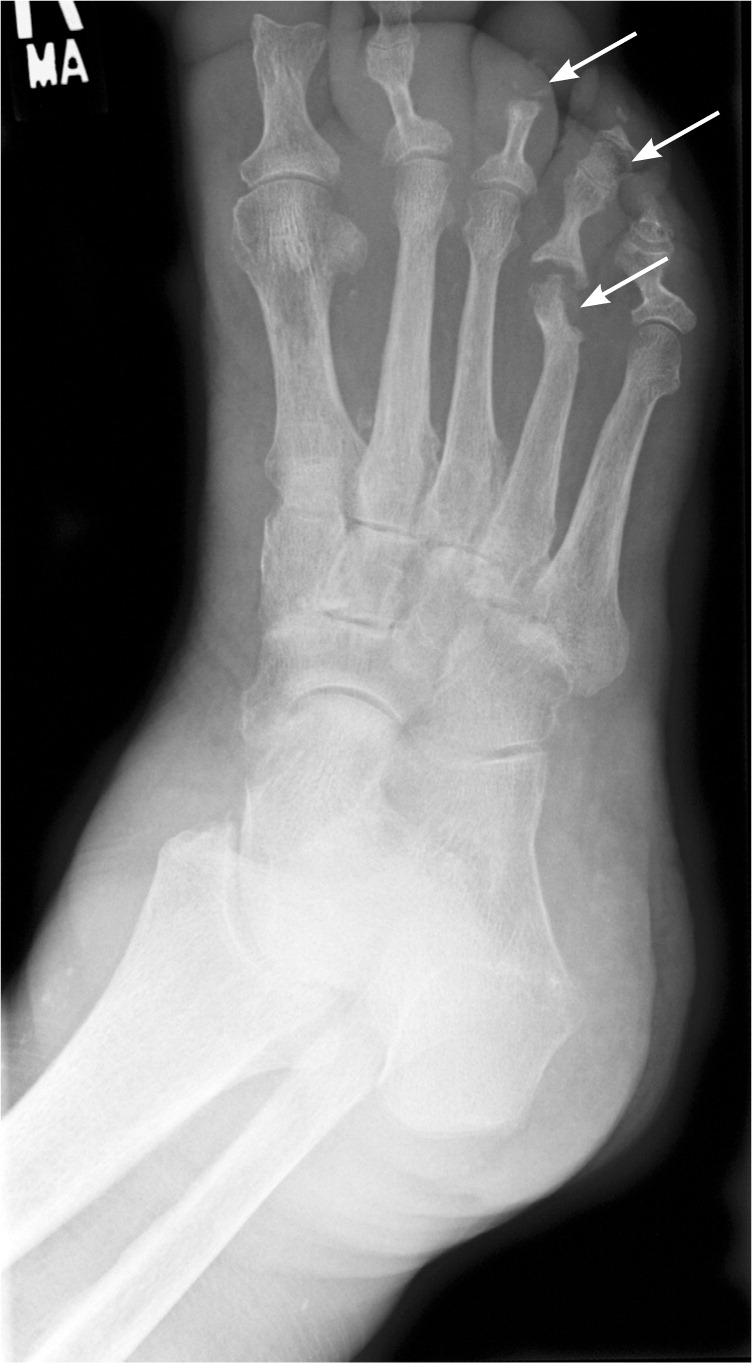
Imaging is useful to characterize the infection and to rule out other potential causes of symptoms. Plain radiography, technetium-99 bone scintigraphy, and magnetic resonance imaging (MRI) are the most useful modalities (Table 224–30 ). Plain radiography usually does not show abnormalities caused by osteomyelitis until about two weeks after the initial infection, when nearly 50 percent of the bone mineral content has been lost.24 Typical findings include non-specific periosteal reaction and osteolysis (Figure 1). Plain radiography is a useful first step that may reveal other diagnoses, such as metastases or osteoporotic fractures. It generally complements information provided by other modalities and should not be omitted, even if more advanced imaging is planned.25
The role of computed tomography in the diagnosis of osteomyelitis is limited. Although computed tomography is superior to MRI in detecting necrotic fragments of bone, its overall value is generally less than that of other imaging modalities. Computed tomography should be used only to determine the extent of bony destruction (especially in the spine), to guide biopsies, or in patients with contraindications to MRI.26
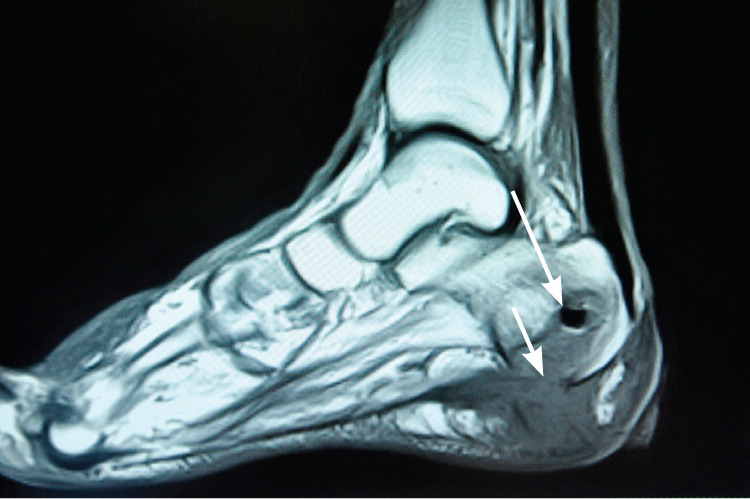
MRI provides better information for early detection of osteomyelitis than do other imaging modalities (Figure 2). MRI can detect osteomyelitis within three to five days of disease onset.24 Most studies of the diagnostic accuracy of MRI in detecting osteomyelitis included patients with diabetic foot ulcers.27 The sensitivity and specificity of MRI in the diagnosis of osteomyelitis may be as high as 90 percent.28,29 Because MRI can also detect necrotic bone, sinus tracts, or abscesses, it is superior to bone scintigraphy in diagnosing and characterizing osteomyelitis.28 Its use can be limited, however, if surgical hardware is present.
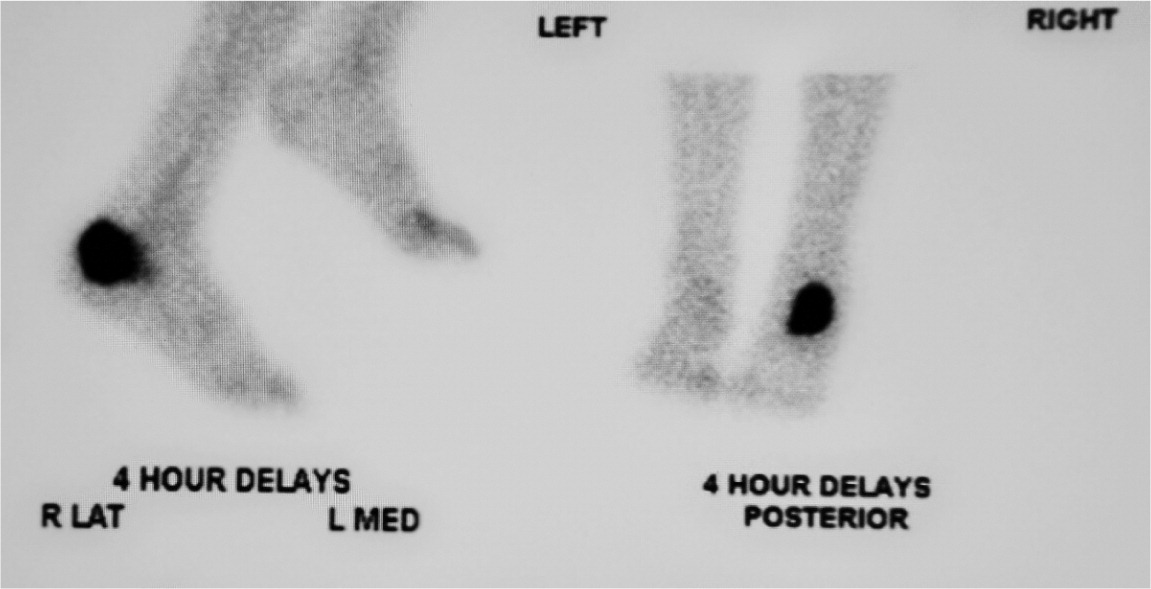
Nuclear imaging can be helpful in diagnosing osteomyelitis (Figure 3). Three-phase technetium-99 bone scintigraphy and leukocyte scintigraphy are usually positive within a few days of the onset of symptoms.24 The sensitivity of bone scintigraphy is comparable to MRI, but the specificity is poor. Leukocyte scintigraphy also has poor specificity, but when combined with three-phase bone scintigraphy, sensitivity and specificity are improved.29 Bone and leukocyte scintigraphy can provide valuable information if MRI is contraindicated or unavailable.30
Other imaging modalities seem promising for the diagnosis of osteomyelitis, but they are not routinely used. Positron emission tomography has the highest sensitivity and specificity—more than 90 percent—but it is expensive and not as widely available as other modalities.29 The role of musculoskeletal ultrasonography in the diagnosis of osteomyelitis is evolving. Some studies suggest that in some patients, such as those with sickle cell disease, detection of subperiosteal fluid collections can be useful or even diagnostic; however, reliable estimates of sensitivity and specificity are lacking.26
Treatment
Treatment of osteomyelitis depends on appropriate antibiotic therapy and often requires surgical removal of infected and necrotic tissue. Choice of antibiotic therapy should be determined by culture and susceptibility results, if possible (Table 3).31,32 In the absence of such information, broad-spectrum, empiric antibiotics should be administered. False-negative blood or biopsy cultures are common in patients who have begun antibiotic therapy. If clinically possible, delaying antibiotics is recommended until microbial culture and sensitivity results are available. Indications for surgery include antibiotic failure, infected surgical hardware, and chronic osteomyelitis with necrotic bone and soft tissue.33
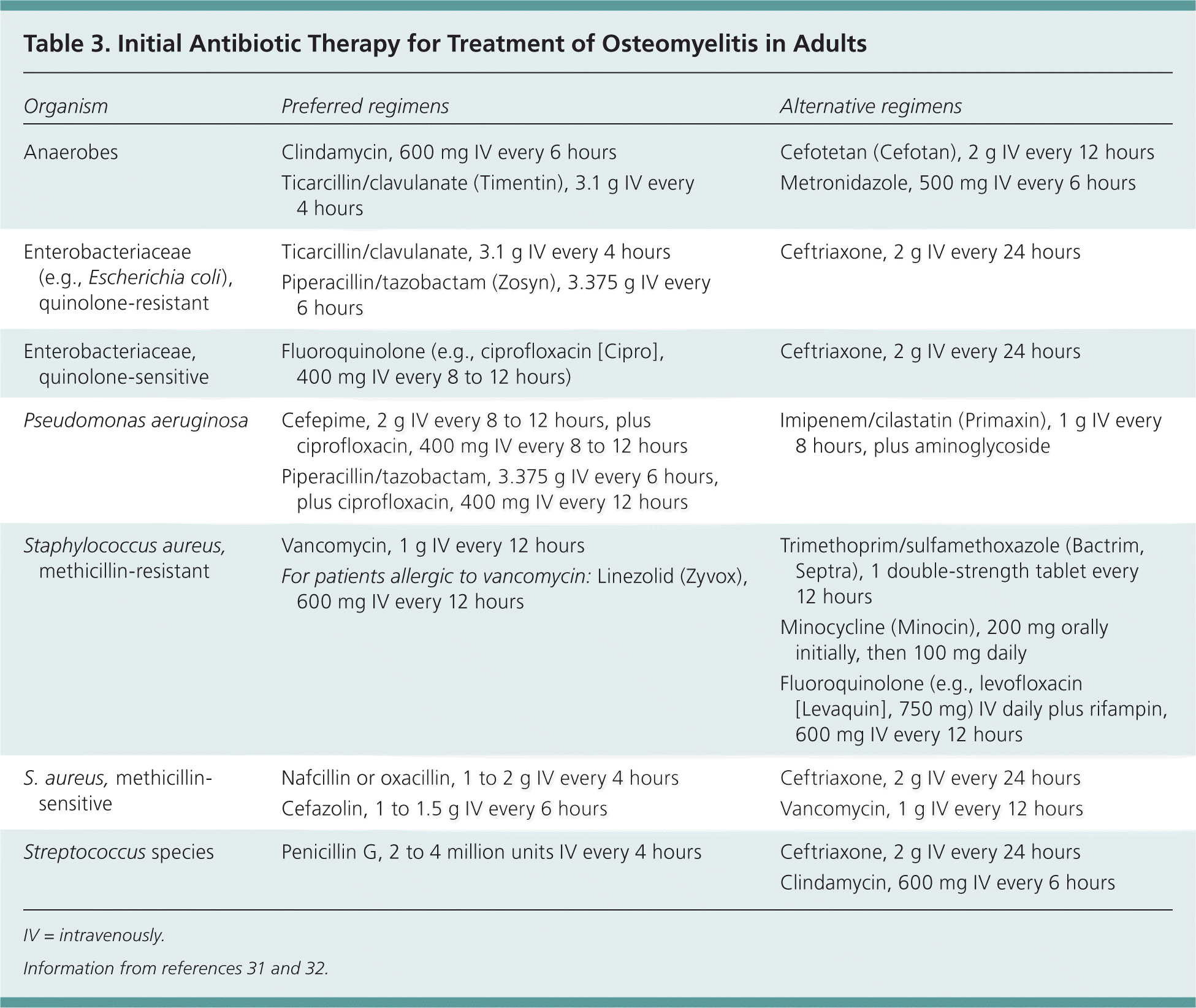
| Organism | Preferred regimens | Alternative regimens |
|---|---|---|
| Anaerobes |
|
|
| Enterobacteriaceae (e.g., Escherichia coli), quinolone-resistant |
|
|
| Enterobacteriaceae, quinolone-sensitive |
|
|
| Pseudomonas aeruginosa |
|
|
| Staphylococcus aureus, methicillin-resistant |
|
|
| S. aureus, methicillinsensitive |
|
|
| Streptococcus species |
|
|
Acute hematogenous osteomyelitis in children typically requires a much shorter course of antibiotic therapy than does chronic osteomyelitis in adults. Although randomized controlled trials are lacking, therapy with four days of parenteral antibiotics followed by oral antibiotics for a total of four weeks seems to prevent recurrence in children who have no serious underlying pathology.34 In immunocompromised children, the transition to oral antibiotics should be delayed, and treatment should continue for at least six weeks based on clinical response.7 Recurrence rates are typically higher in this population. Surgical treatment in immunocompetent children is rare.
Despite the use of surgical debridement and long-term antibiotic therapy, the recurrence rate of chronic osteomyelitis in adults is about 30 percent at 12 months.35 Recurrence rates in cases involving P. aeruginosa are even higher, nearing 50 percent. The optimal duration of antibiotic treatment and route of delivery are unclear.36 For chronic osteomyelitis, parenteral antibiotic therapy for two to six weeks is generally recommended, with a transition to oral antibiotics for a total treatment period of four to eight weeks.31 Long-term parenteral therapy is likely as effective as transitioning to oral medications, but has similar recurrence rates with increased adverse effects.31,36 In some cases, surgery is necessary to preserve viable tissue and prevent recurrent systemic infection.
Antibiotic regimens for the empiric treatment of acute osteomyelitis, particularly in children, should include an agent directed against S. aureus. Betalactam antibiotics are first-line options unless MRSA is suspected. If methicillin resistance among community isolates of Staphylococcus is greater than 10 percent, MRSA should be considered in initial antibiotic coverage.34 Intravenous vancomycin is the first-line choice. In patients with diabetic foot infections or penicillin allergies, fluoroquinolones are an alternate option for staphylococcal infections; these agents seem to be as effective as beta-lactams.32 Fluoroquinolones also cover quinolone-sensitive enterobacteria and other gram-negative rods.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms osteomyelitis, imaging, diagnosis, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, the Cochrane database, the Database of Abstracts of Reviews of Effects, the National Guideline Clearinghouse, and Dynamed. Search date: June 2, 2010.