Am Fam Physician. 2011;84(9):995-1002
Author disclosure: No relevant financial affiliations to disclose.
Shave and punch biopsies are essential procedures for physicians who manage skin conditions. These office-based procedures can diagnose questionable dermatologic lesions, including possible malignancies. Approaches include the superficial shave biopsy, saucerization excision, punch biopsy, and elliptical excision. A superficial shave biopsy can be used for raised lesions. A saucerization biopsy may be performed for flat or pigmented lesions. Punch biopsies yield full-thickness samples and can be used for lesions that require dermal or subcutaneous tissue for diagnosis. Indications for biopsy of suspected melanoma remain controversial. Sufficient tissue may be obtained with the quicker, less costly saucerization biopsy or the more time-consuming, invasive elliptical excisional biopsy.
Skin biopsies are simple office procedures that can provide useful information about undiagnosed lesions such as neoplasms, bullous disorders, keratoses, or dysplastic nevi. A diagnostic biopsy can also be the definitive treatment for some malignant, irritated, or precancerous lesions. The primary types of skin biopsies are incisional and excisional. Incisional biopsies sample only part of the lesion (e.g., superficial shave, partial punch), whereas excisional biopsies remove the entire lesion for diagnostic purposes (e.g., fusiform ellipse, punch for 1- to 4-mm lesions, saucerization [also called deep scoop shave]).1
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Whenever possible, lesions should be excised for diagnostic purposes using narrow margins. | C | 4, 5, 22 | Consensus guidelines |
| Punch or superficial shave biopsies may be more appropriate in carefully selected clinical circumstances (e.g., for large lesions, when melanoma suspicion is low) because of their potential effects on staging and prognosis. | C | 3, 7, 9–13, 16 | Consensus guidelines |
| A suspected melanoma should be excised using a 1- to 3-mm margin. | C | 3, 4 | Consensus guidelines |
| The type of biopsy performed does not influence survival rates in patients with melanoma. | B | 5, 10–12, 14–16 | Total of 5,240 patients in seven outcome case-control series |
This article reviews conditions that warrant office-based biopsy; the types of biopsies commonly performed in the office setting; and indications (Table 12–4), contraindications, and proper techniques. Superficial shave, saucerization, and punch biopsies are emphasized. Elliptical excisions and newer biopsy techniques, such as the Ellman unit or radiofrequency surgery, are beyond the scope of this article.
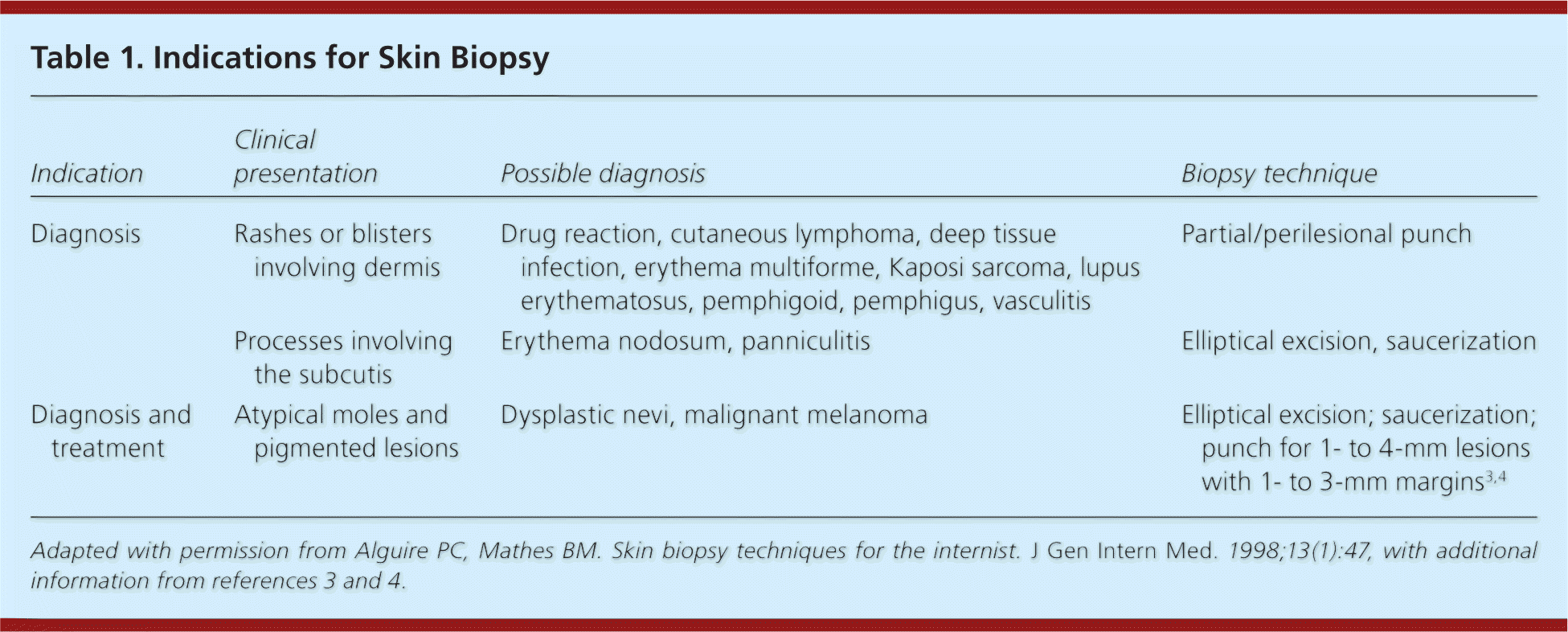
| Indication | Clinical presentation | Possible diagnosis | Biopsy technique |
|---|---|---|---|
| Diagnosis | Rashes or blisters involving dermis | Drug reaction, cutaneous lymphoma, deep tissue infection, erythema multiforme, Kaposi sarcoma, lupus erythematosus, pemphigoid, pemphigus, vasculitis | Partial/perilesional punch |
| Processes involving the subcutis | Erythema nodosum, panniculitis | Elliptical excision, saucerization | |
| Diagnosis and treatment | Atypical moles and pigmented lesions | Dysplastic nevi, malignant melanoma | Elliptical excision; saucerization; punch for 1- to 4-mm lesions with 1- to 3-mm margins3,4 |
Choosing the Biopsy Procedure
SHAVE
Shave biopsy is the most commonly used technique because of how quickly it can be performed, the simplicity of wound care, cosmesis, and cost-effectiveness.5
Superficial Shave Biopsy. A superficial shave biopsy is used for lesions that are predominantly epidermal without extension into the dermis, such as warts, papillomas, skin tags, superficial basal or squamous cell carcinomas, and seborrheic or actinic keratoses.2,6 This type of biopsy is not appropriate for suspicious pigmented lesions.2,6,7 A superficial shave removes a thin disk of tissue, typically by scalpel (generally a no. 15 blade), although many physicians prefer a Dermablade, a double-edged razor blade, or scissors1,2,6,8,9 (Figure 1 and Figure 2). This procedure yields a flat, thin specimen of combined epidermis and upper dermis (less than 1 mm).9 Hemostasis is usually obtained with aluminum chloride 20% solution; silver nitrate or Monsel solution (ferric subsulfate) may be used instead, but staining can occur.2 Keeping the area covered and moist for at least one week may decrease scarring. Shave biopsy on the foot is preferable to a technique requiring sutures because of excessive tension.
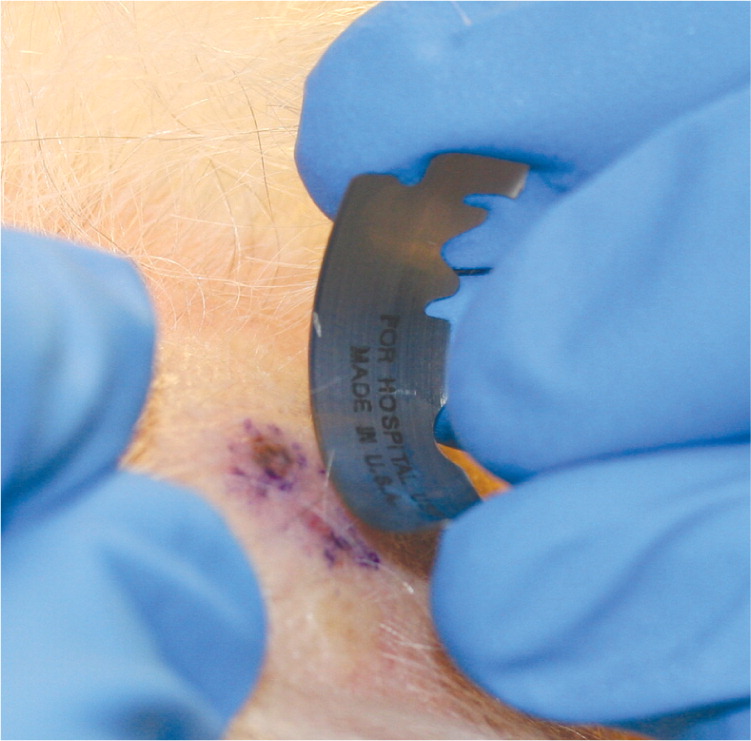
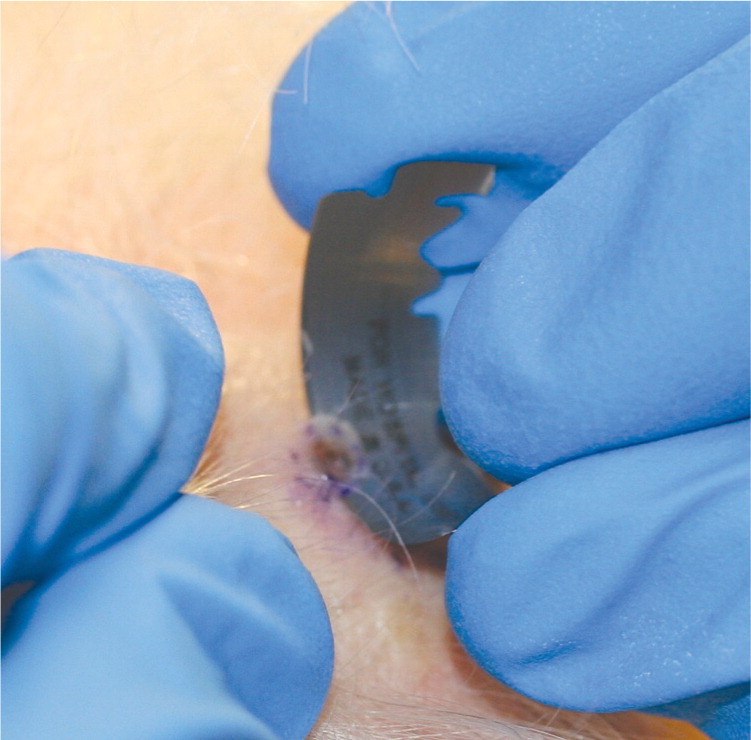
Saucerization Biopsy. In a saucerization biopsy, a thick disk of tissue is removed with a curved blade, yielding a specimen that extends to at least the mid-dermis or subcutaneous fat (1 to 4 mm deep).5,9 Saucerization is considered an excisional biopsy.1,8,9 These biopsies may be indicated in patients with wider pigmented lesions or in those with lesions that are difficult to remove with an elliptical excision because of cosmesis or anatomic location. For example, the upper back, shoulders, anterior chest, upper arms, lower extremities, and ears are areas where scars may become hypertrophic and spread over time.1 Compared with elliptical excision, saucerization leaves a smaller, rounder, more cosmetically acceptable scar.1
Although a full-thickness elliptical excision biopsy has conventionally been recommended for suspicious skin lesions,1,2,5–7,9–13 numerous retrospective reviews with a total of 5,240 patients have shown that saucerization biopsies can be the procedure of choice and do not statistically affect survival rates for malignant lesions.10–13,14–16 A saucerization biopsy is quicker, less invasive, less costly, and more cosmetically appealing, and it generally can provide as much diagnostic tissue as an elliptical excision.8–10,12,13,17
PUNCH
In a punch biopsy, a skin punch instrument with a circular blade extends through to the subcutaneous fat to obtain a cylindrical specimen.1 Punch biopsies can be excisional or incisional, depending on the size of the lesion and the type of tissue that needs to be obtained. Punch biopsies may be used for lesions that require dermal or subcutaneous tissue for diagnosis, including inflammatory or bullous lesions, dysplastic or complex nevi that are too large to be excised, panniculitis, and scalp or hair follicle biopsies.6 Bullae should be biopsied perilesionally; if neoplasm is suspected, the biopsy should be performed on the thickest area of the lesion that can be obtained with narrow margins.2,5 The site should be closed with simple interrupted or vertical mattress sutures to provide the best cosmetic result (Table 218–20).1 Secondary intention healing and suturing have similar cosmetic results for lesions 1 to 4 mm in diameter.18,21 Limitations of the punch biopsy are that it may not provide a wide enough sample in suspect pigmented lesions (because of the narrow, deep nature of the specimen13), which in turn may affect tumor staging and prognosis.14,16
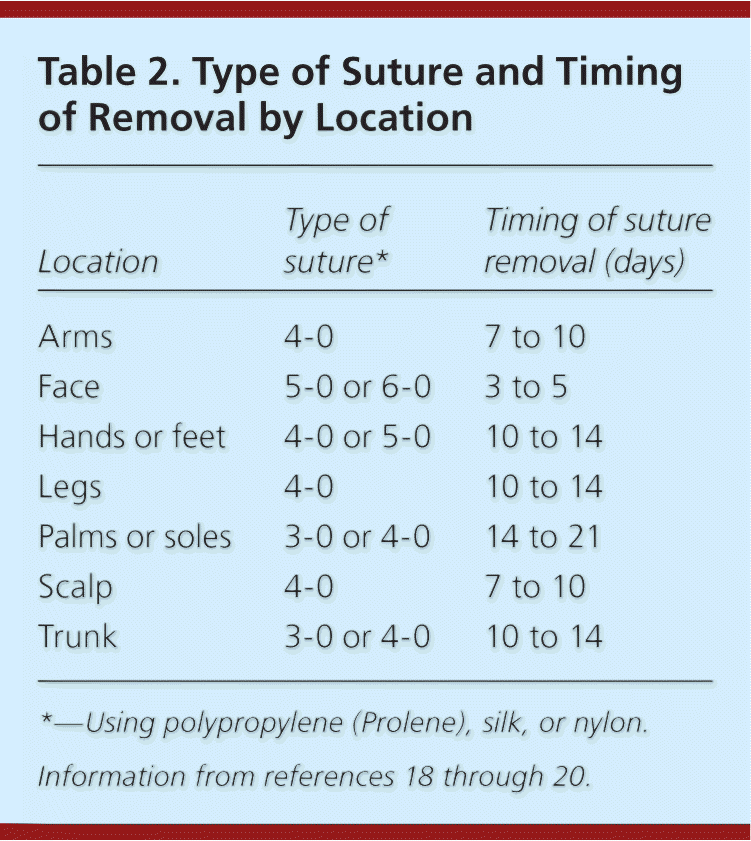
| Location | Type of suture* | Timing of suture removal (days) |
|---|---|---|
| Arms | 4-0 | 7 to 10 |
| Face | 5-0 or 6-0 | 3 to 5 |
| Hands or feet | 4-0 or 5-0 | 10 to 14 |
| Legs | 4-0 | 10 to 14 |
| Palms or soles | 3-0 or 4-0 | 14 to 21 |
| Scalp | 4-0 | 7 to 10 |
| Trunk | 3-0 or 4-0 | 10 to 14 |
Special Considerations: Lesions Suspicious for Melanoma
A saucerization, punch (for lesions smaller than 4 mm), or elliptical excision biopsy may be performed when a lesion is suspicious for melanoma. A thorough history for risk factors should be obtained, and the lesion should be inspected using the ABCDE criteria (asymmetry, border, color, diameter, evolution; Table 322, Figure 3), the Glasgow 7-point checklist, and the “ugly duckling” sign (i.e., one lesion that is distinct from others).5 Factors to identify patients at high risk of malignancy include a personal or family history of melanoma, fair complexion, presence of multiple pigmented nevi, a history of numerous severe sunburns, and advanced age.1,5 Lesions that fulfill some or all of the ABCDE criteria can be biopsied. The American Academy of Dermatology Guidelines/Outcomes Committee for Cutaneous Melanoma and the National Comprehensive Cancer Network recommend initial excision with 1- to 3-mm margins for suspect lesions3,4; other biopsy methods can be used, including punch and full-thickness incisional biopsies in specific anatomic areas or for large lesions that are difficult to excise.5 The best biopsy method for suspected

| ABCDE system | |
| Geometric Asymmetry in two axes | |
| Irregular Border | |
| At least two different Colors in lesion | |
| Maximum Diameter > 6 mm | |
| Evolution of lesion | |
| Glasgow 7-point checklist | |
| Major features | |
| Change in size of lesion | |
| Irregular border | |
| Irregular pigmentation | |
| Minor features | |
| Inflammation | |
| Itch or altered sensation | |
| Lesion larger than others | |
| Oozing and crusting | |
melanoma is controversial. Studies in the 1980s and early 1990s suggested that incisional biopsies into pigmented lesions may affect the outcome of melanoma and increase the spread of abnormal cells.1,5,11 However, in the past 10 years, numerous reviews have shown that the type of biopsy does not negatively influence melanoma survival rates.5,10–12,14–16 Punch and superficial shave biopsies may have greater diagnostic inaccuracy and can affect subsequent tumor staging, prognosis, and follow-up recommendations compared with saucerization and elliptical excision biopsies.3,7,9–13,16 Up to 20 percent of initial partial biopsies for melanoma are misleading because they underestimate the final Breslow depth.9 Thus, excisional biopsies should be performed whenever possible for lesions suspicious for melanoma.3,4 Saucerization biopsy (an excisional biopsy) is an important tool for physicians without training or resources to provide an elliptical excision biopsy.8,10
Materials and Method
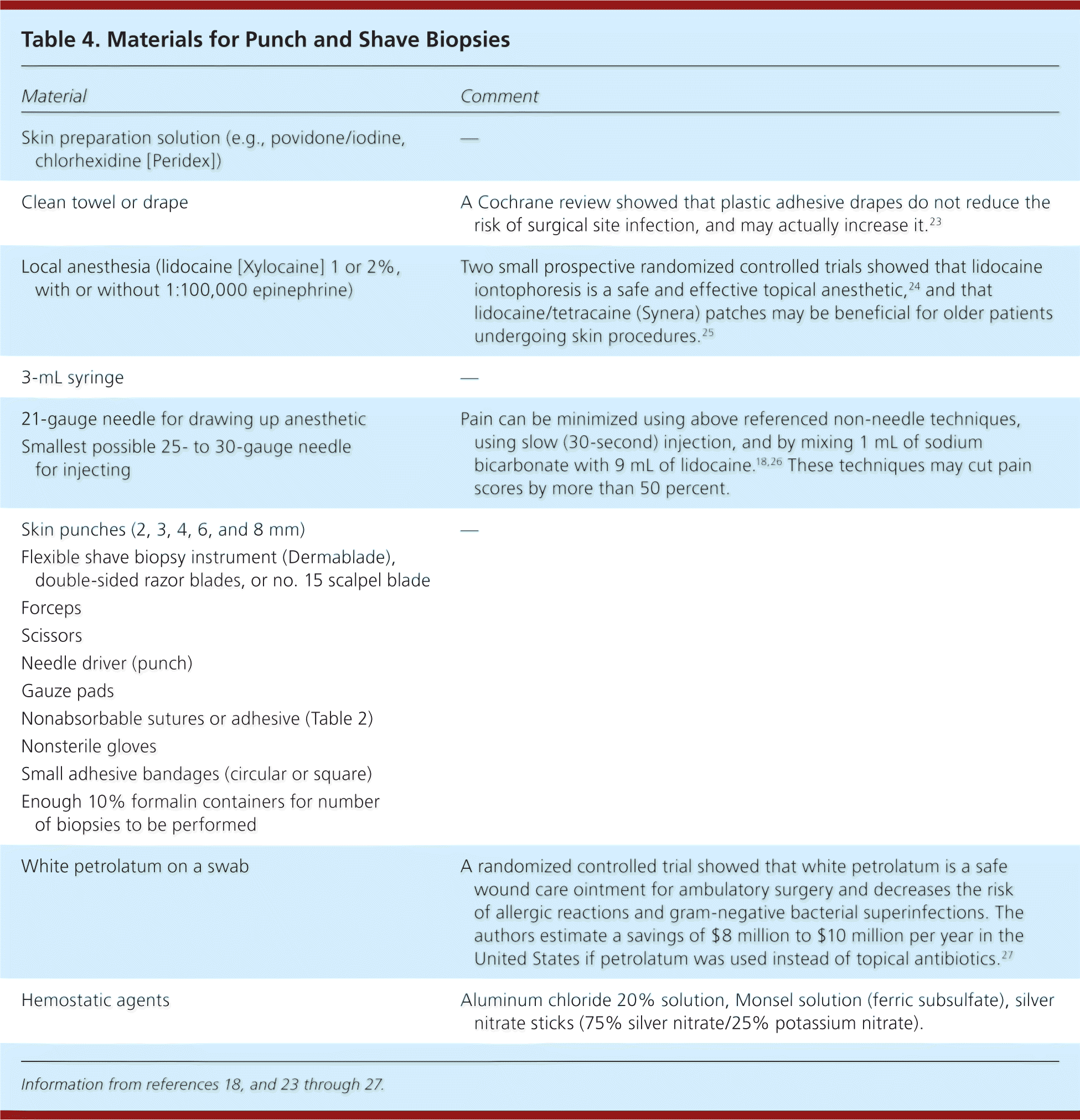
| Material | Comment |
|---|---|
| Skin preparation solution (e.g., povidone/iodine, chlorhexidine [Peridex]) | — |
| Clean towel or drape | A Cochrane review showed that plastic adhesive drapes do not reduce the risk of surgical site infection, and may actually increase it.23 |
| Local anesthesia (lidocaine [Xylocaine] 1 or 2%, with or without 1:100,000 epinephrine) | Two small prospective randomized controlled trials showed that lidocaine iontophoresis is a safe and effective topical anesthetic,24 and that lidocaine/tetracaine (Synera) patches may be beneficial for older patients undergoing skin procedures.25 |
| 3-mL syringe | — |
| 21-gauge needle for drawing up anesthetic Smallest possible 25- to 30-gauge needle for injecting | Pain can be minimized using above referenced non-needle techniques, using slow (30-second) injection, and by mixing 1 mL of sodium bicarbonate with 9 mL of lidocaine.18,26 These techniques may cut pain scores by more than 50 percent. |
| Skin punches (2, 3, 4, 6, and 8 mm) | — |
| Flexible shave biopsy instrument (Dermablade), double-sided razor blades, or no. 15 scalpel blade | |
| Forceps | |
| Scissors | |
| Needle driver (punch) | |
| Gauze pads | |
| Nonabsorbable sutures or adhesive (Table 2) | |
| Nonsterile gloves | |
| Small adhesive bandages (circular or square) | |
| Enough 10% formalin containers for number of biopsies to be performed | |
| White petrolatum on a swab | A randomized controlled trial showed that white petrolatum is a safe wound care ointment for ambulatory surgery and decreases the risk of allergic reactions and gram-negative bacterial superinfections. The authors estimate a savings of $8 million to $10 million per year in the United States if petrolatum was used instead of topical antibiotics.27 |
| Hemostatic agents | Aluminum chloride 20% solution, Monsel solution (ferric subsulfate), silver nitrate sticks (75% silver nitrate/25% potassium nitrate). |

| Obtain consent. | |
| Clean skin. | |
| Anesthetize skin. | |
| Superficial shave: | |
| For macular or raised nonsuspicious lesions, hold blade parallel to the skin and shallowly remove a thin disk or the lesion itself, if raised (Figures 1 and 2). | |
| Saucerization: | |
| For pigmented lesions, measure a 1- to 3-mm margin before shaving3,4 (Figure 4A; also see http://www.youtube.com/user/AFPJournal#p/a/u/1/R0yvX-ty9VM [scoop shave biopsy–long version] and http://www.youtube.com/user/AFPJournal#p/a/u/2/bCrRr1s3wCI [scoop shave biopsy–short version]). | |
| Anesthetize, creating a wheal to make the lesion easier to shave (Figure 4B), and squeeze skin between the thumb and forefinger of the nondominant hand to further elevate the lesion.1,6 | |
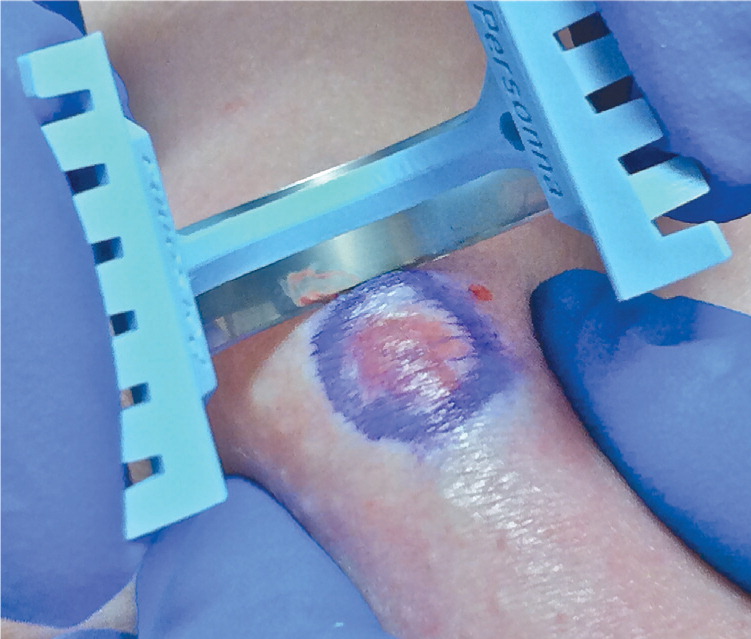
| Hold blade at a 45-degree angle to the skin, bend or bow the blade depending on the width of lesion, and remove a disk of tissue well into the subcutaneous fat1,8 (Figure 5). | |
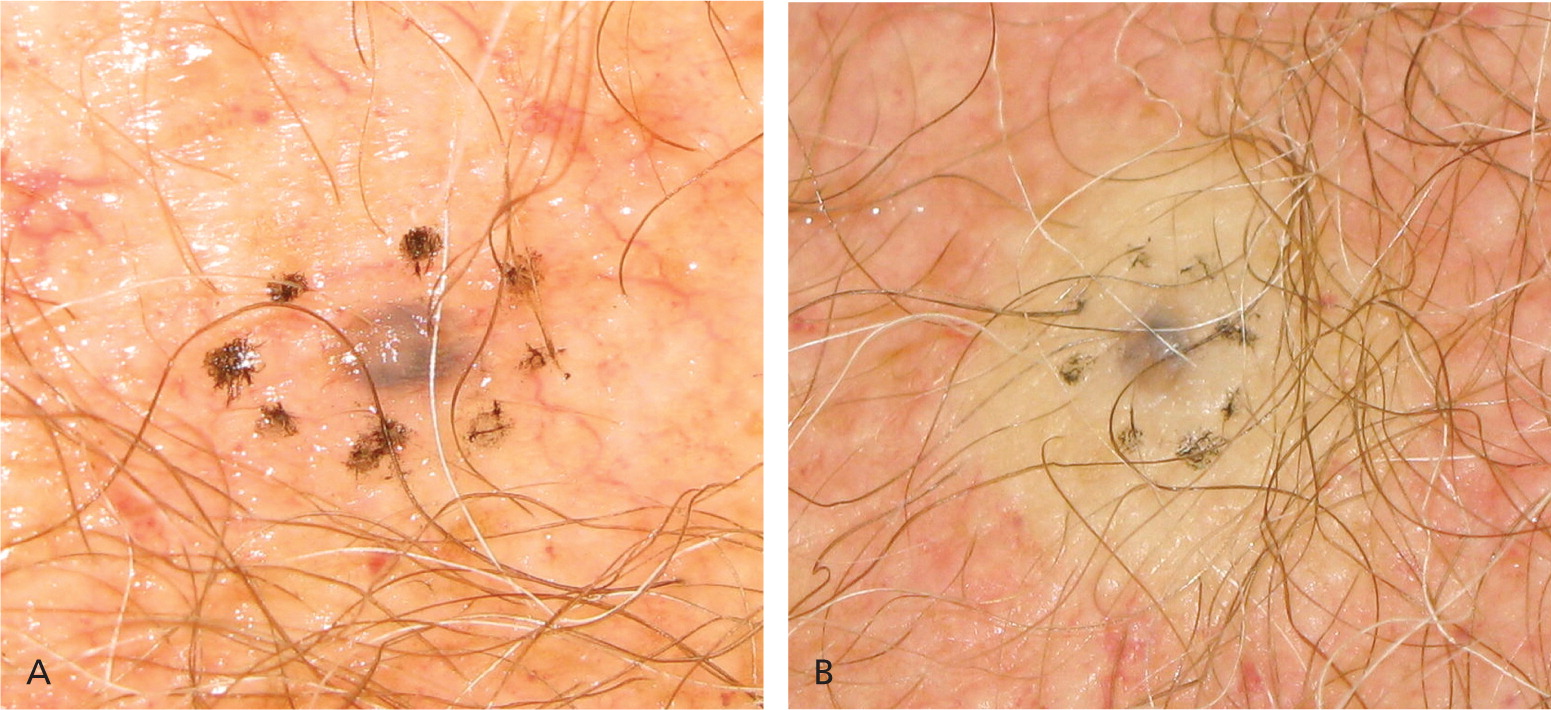
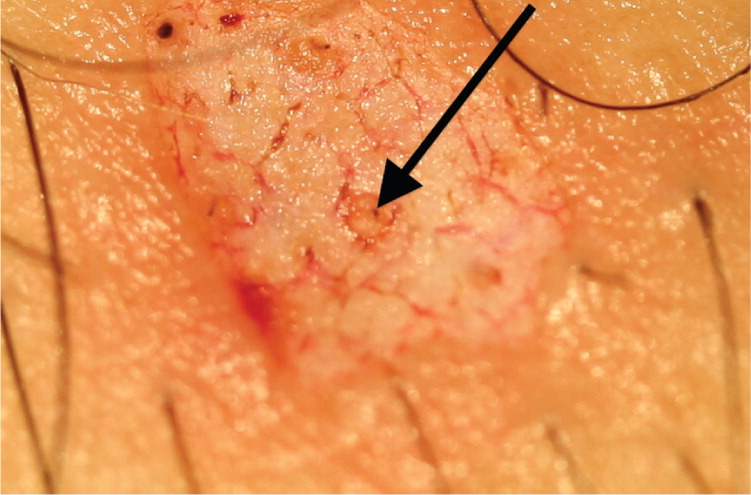
| If a nidus of pigment remains after saucerization, a punch or elliptical biopsy must be performed, and the sample sent in the same specimen container (Figure 6). | |
| Use a hemostatic agent or electrocautery and wipe clean. | |
| Dress with petrolatum and instruct the patient to keep the area moist and covered for at least one week to minimize scarring. | |

| Obtain consent. |
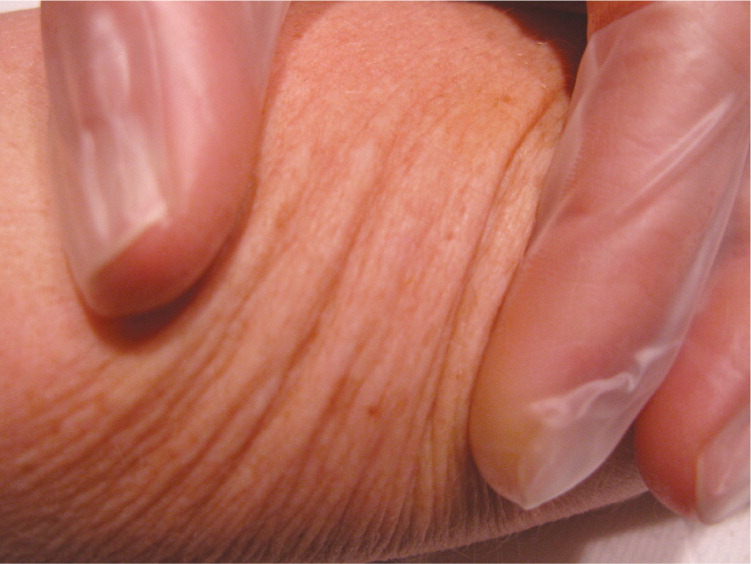
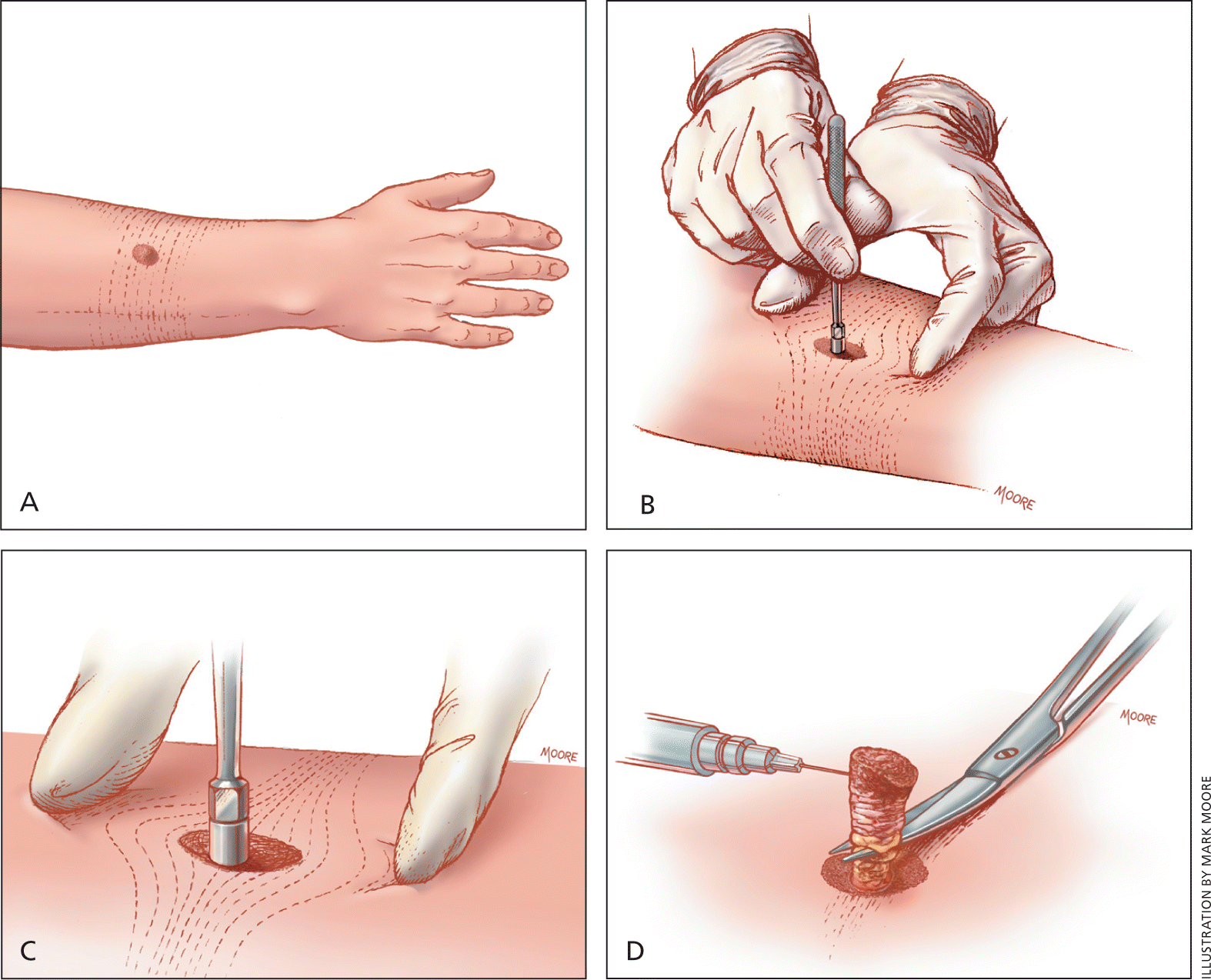
| Determine skin line orientation using Langer lines (Figures 7 and 8). If pinching in one direction produces few lines and another produces numerous, pull skin perpendicular to the numerous lines to punch; this will produce an oval that can be easily approximated6 (Figures 8, 9, and 10). |
| Determine the punch size with a 1- to 3-mm margin around the lesion. If area is too large for a punch biopsy, consider a larger saucerization biopsy5 (Table 5). |
| Prepare skin. |
| Drape skin. |
| Anesthetize, creating a wheal (Figure 4B). |
| Place punch over lesion after pulling perpendicular to Langer lines, and firmly but gently rotate through skin until there is a decrease in tension on the tissue; this indicates a full-thickness sample. |
| Remove tissue gently with forceps or needle to minimize crush artifact (Figure 10). |
| If sample area is 4 mm or less, may dab with aluminum chloride 20% solution or other hemostatic agent (Table 4) or close with one suture (Table 2); may also consider closing with surgical adhesive. A Cochrane review showed that tissue adhesives are as effective as traditional sutures for closure of general incisions without high tension.28 |
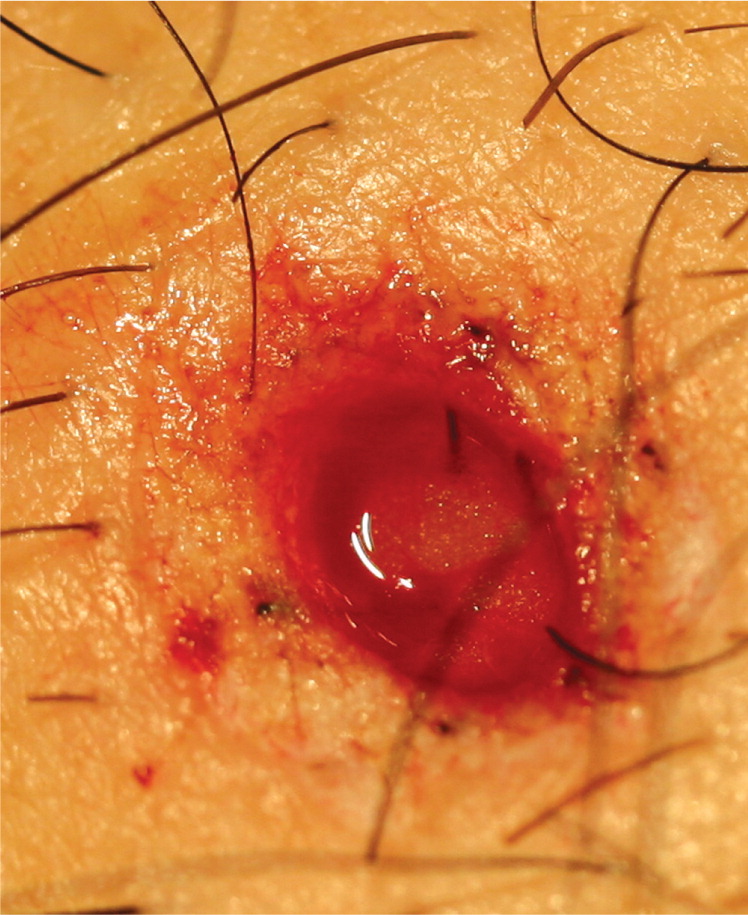
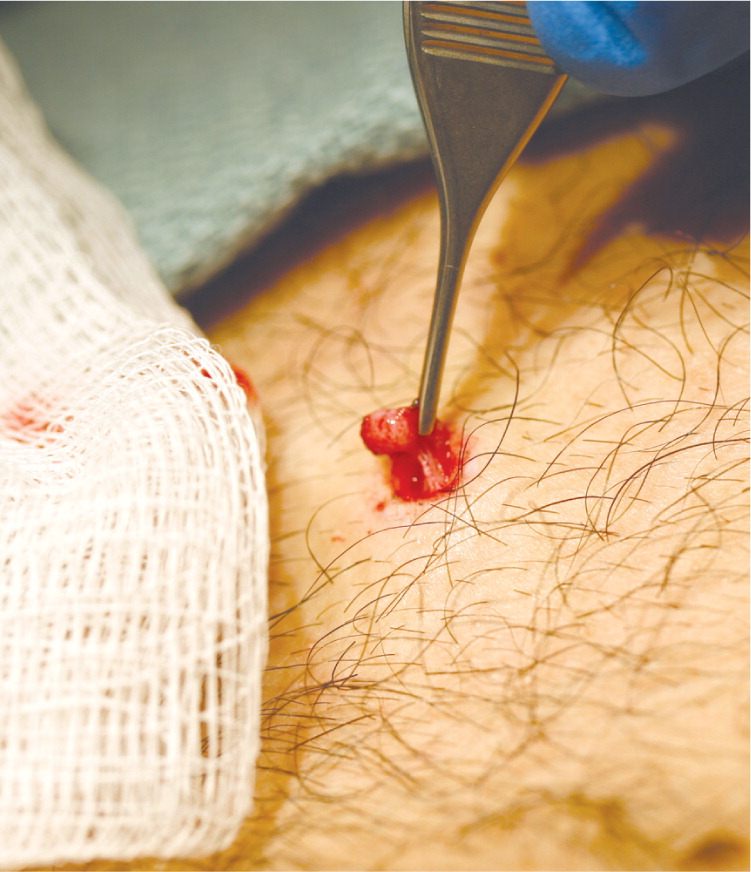

| Clean area after hemostasis is achieved. |
| Cover with petrolatum and sterile dressing. |
| Send tissue to pathology in formalin; if specimen is large enough and there is high suspicion for malignancy, consider suture tagging an area for pathologist. |
| Give discharge instructions; keep area covered and dry for 24 hours (punch biopsies) or covered and moist for at least one week (shave biopsies). |
| Complications are rare; bleeding can be managed with pressure, suture, or cautery; if infection occurs, it usually appears within three days after biopsy and can be treated with suture removal or oral antibiotics.18 |
Referral may be considered for patients with lesions on the eyelids, nose, palms, or soles.2 Physicians should use caution in patients who have skin allergies to topical treatments, who have bleeding disorders, or who are taking medications that may interfere with hemostasis. Patients on aspirin therapy generally can be treated with careful attention to hemostasis and pressure dressings.
See a short-version video and the long-version video on scoop shave skin biopsy.
Scoop Shave Skin Biopsy (Short Version)
Scoop Shave Skin Biopsy (Long Version)
Data Sources: A PubMed search was completed in Clinical Queries using the key terms shave, biopsy, and punch. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Bandolier, Canadian Task Force on Preventive Health Care, Clinical Evidence, the Cochrane database, Database of Abstracts of Reviews of Effects, Effective Health Care, National Center for Complementary and Alternative Medicine, the Institute for Clinical Systems Improvement, U.S. Preventive Services Task Force, the National Guideline Clearinghouse database, Essential Evidence Plus, and UpToDate. Search dates: June 1 through 6, 2010; March 2011; and September 2011.