Am Fam Physician. 2011;84(11):1208-1210
Author disclosure: No relevant financial affiliations to disclose.
Prolotherapy is an injection-based complementary therapy for common chronic musculoskeletal conditions including tendinopathy, knee osteoarthritis, and low back pain.1 It involves the injection of irritant solutions into tender ligamentous and tendinous attachments and adjacent joint spaces. Central to the application of prolotherapy is the premise that chronic musculoskeletal pain and disability often result from degeneration associated with these structures, and that prolotherapy addresses this degeneration at the tissue level. Although the mechanism of action for prolotherapy is not clearly understood, recent animal model studies reported that prolotherapy is associated with local inflammation, which may lead to induction of tissue growth factors.2 Prolotherapy injections may also act as central pain modulators.1
Although prolotherapy techniques and injected solutions vary by condition, clinical severity, and physician preferences, a core principle is that a small volume (0.2 to 0.5 mL) of solution is injected into tender ligamentous and tendinous attachments in a peppering fashion, and into adjacent joint spaces.1 The most common injectant is dextrose 15% (3 mL dextrose 50%, 5 mL saline 0.9%, and 2 mL lidocaine 2% [Xylocaine]); a similar volume of the sclerosant morrhuate sodium is also used.3 Treatment typically involves at least three injection sessions one month apart, but injection intervals vary from two to six weeks.1
Since the mid-1980s, the quantity and quality of clinical prolotherapy research has increased, with a shift from case reports to randomized controlled trials (RCTs) for chronic tendinopathy, knee osteoarthritis, and low back pain.1,4 The strongest data supporting the effectiveness of prolotherapy for any musculoskeletal condition, compared with saline control injections, are for severe refractory lateral epicondylosis. Participants treated with prolotherapy (n = 10) reported approximately a 90 percent reduction in resting elbow pain on a 10-point visual analog scale compared with a 22 percent reduction in the control group (n = 10) at baseline and 16 weeks (absolute effect size of 68 percent; P < .01). Participants in the prolotherapy group also demonstrated improved isometric strength (P < .05) at 16 and 52 weeks compared with the control group.3
In limited studies, other overuse injuries have responded well to prolotherapy, including Achilles5 and adductor tendinopathies,6 and plantar fasciitis.7 Prolotherapy has also been used in multidisciplinary care plans. Participants in an Achilles tendinopathy study responded earlier and with less money spent on treatment when physical therapy and prolotherapy were combined compared with either treatment alone.5
The data supporting prolotherapy for knee osteoarthritis are less clear. In one RCT, participants in the prolotherapy and sham injection groups reported substantial improvements in pain and functional measures compared with baseline measurements; however, there were no statistically significant differences between groups.8 A National Institutes of Health–sponsored pilot-level study (n = 36) assessing prolotherapy for patients with moderate to severe knee osteoarthritis at 52 weeks reported a 36 percent improvement in knee-related quality of life compared with baseline status on the validated Western Ontario and McMaster Universities Osteoarthritis Index.9
The largest and most methodologically rigorous study compared prolotherapy with control (saline) injections in 110 participants with an average of 14 years of nonsurgical low back pain.10 Participants reported substantial and sustained reductions in pain (26 to 44 percent) and disability (30 to 44 percent) at 12 months. However, the reductions in pain and disability were not statistically significant between groups. These findings were contrary to two previous RCTs of lower quality that reported statistically significant positive findings.11,12
Following the contrasting, suggestive findings in low back pain studies, researchers have begun to assess prolotherapy in patients with more clearly diagnosed forms of low back pain in an effort to determine specific low back indications for which this treatment may be effective. Positive outcomes have been reported in prospective studies assessing prolotherapy for refractory coccygodynia,13 sacroiliac joint dysfunction,14 and leg pain caused by moderate to severe degenerative disk disease.15
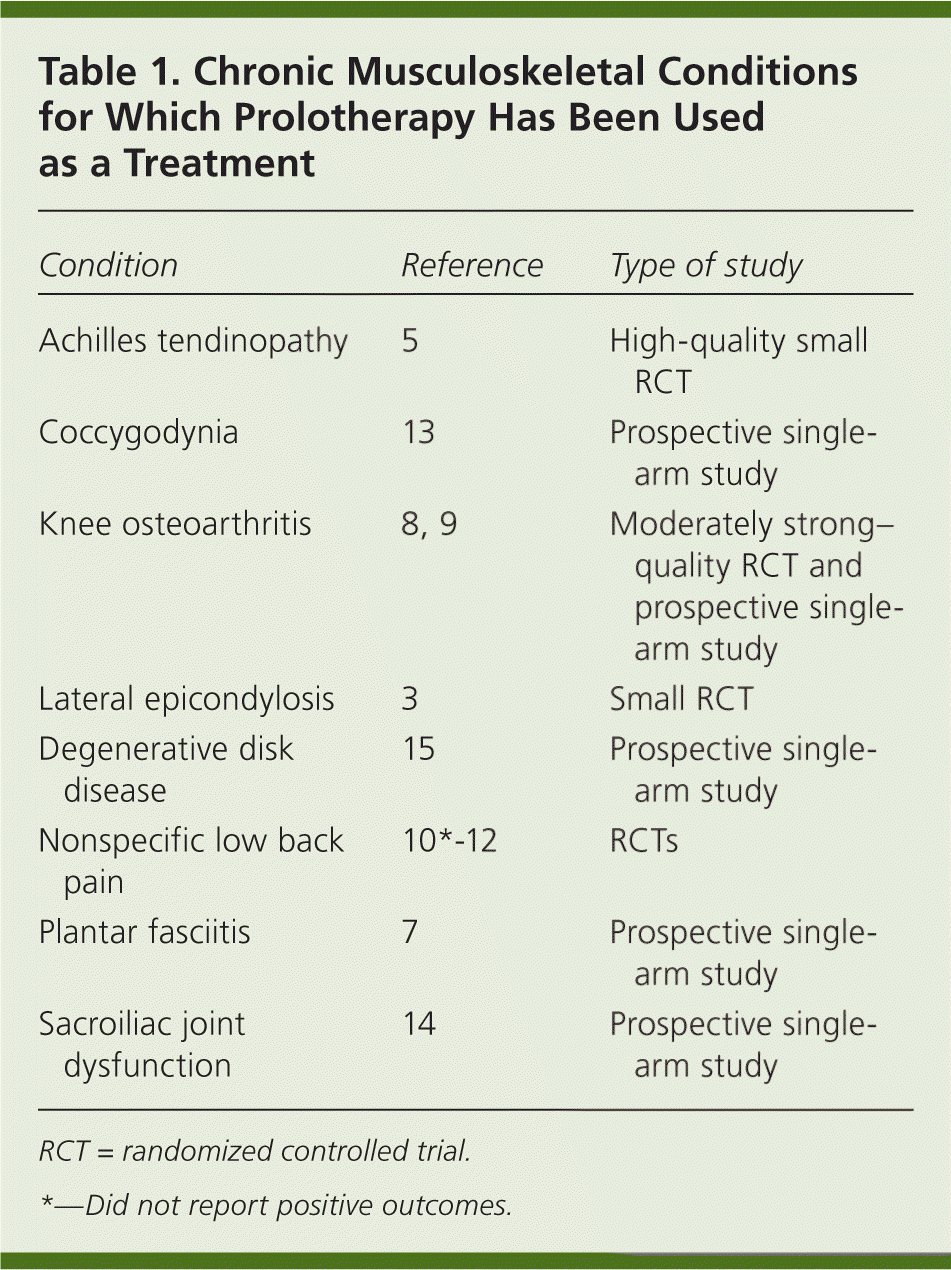
Prolotherapy performed by an experienced injector appears safe; no clinical trials report significant adverse events. Current data suggest that prolotherapy has a positive effect compared with baseline status, and in some cases compared with control therapy, in carefully selected patients for several indications (Table 13,5,7–15 ). However, a more complete understanding of its clinical application will require more research. Prolotherapy is the subject of ongoing federally funded RCTs in the United States16,17 and Canada.18
| Condition | Reference | Type of study |
|---|---|---|
| Achilles tendinopathy | 5 | High-quality small RCT |
| Coccygodynia | 13 | Prospective singlearm study |
| Knee osteoarthritis | 8, 9 | Moderately strong–quality RCT and prospective singlearm study |
| Lateral epicondylosis | 3 | Small RCT |
| Degenerative disk disease | 15 | Prospective singlearm study |
| Nonspecific low back pain | 10*-12 | RCTs |
| Plantar fasciitis | 7 | Prospective singlearm study |
| Sacroiliac joint dysfunction | 14 | Prospective singlearm study |