Am Fam Physician. 2012;85(1):35-43
A more recent article on abnormal uterine bleeding in premenopausal women is available.
Patient information: See related handout on abnormal uterine bleeding, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Up to 14 percent of women experience irregular or excessively heavy menstrual bleeding. This abnormal uterine bleeding generally can be divided into anovulatory and ovulatory patterns. Chronic anovulation can lead to irregular bleeding, prolonged unopposed estrogen stimulation of the endometrium, and increased risk of endometrial cancer. Causes include polycystic ovary syndrome, uncontrolled diabetes mellitus, thyroid dysfunction, hyperprolactinemia, and use of antipsychotics or antiepileptics. Women 35 years or older with recurrent anovulation, women younger than 35 years with risk factors for endometrial cancer, and women with excessive bleeding unresponsive to medical therapy should undergo endometrial biopsy. Treatment with combination oral contraceptives or progestins may regulate menstrual cycles. Histologic findings of hyperplasia without atypia may be treated with cyclic or continuous progestin. Women who have hyperplasia with atypia or adenocarcinoma should be referred to a gynecologist or gynecologic oncologist, respectively. Ovulatory abnormal uterine bleeding, or menorrhagia, may be caused by thyroid dysfunction, coagulation defects (most commonly von Willebrand disease), endometrial polyps, and submucosal fibroids. Transvaginal ultrasonography or saline infusion sonohysterography may be used to evaluate menorrhagia. The levonorgestrel-releasing intrauterine system is an effective treatment for menorrhagia. Oral progesterone for 21 days per month and nonsteroidal anti-inflammatory drugs are also effective. Tranexamic acid is approved by the U.S. Food and Drug Administration for the treatment of ovulatory bleeding, but is expensive. When clear structural causes are identified or medical management is ineffective, polypectomy, fibroidectomy, uterine artery embolization, and endometrial ablation may be considered. Hysterectomy is the most definitive treatment.
Abnormal uterine bleeding occurs in 9 to 14 percent of women between menarche and menopause, significantly impacting quality of life and imposing financial burden.1 The etiologies and treatments for abnormal uterine bleeding over the reproductive years are best understood in the context of normal menstrual physiology. A normal cycle starts when pituitary follicle-stimulating hormone induces ovarian follicles to produce estrogen. Estrogen stimulates proliferation of the endometrium. A luteinizing hormone surge prompts ovulation; the resultant corpus luteum produces progesterone, inducing a secretory endometrium. In the absence of pregnancy, estrogen and progesterone levels decline, and withdrawal bleeding occurs 13 to 15 days postovulation.2 Disruption of normal physiology, anatomic changes in the endometrium, or endometrial cancer may result in abnormal uterine bleeding. Genital bleeding during childhood, uterine bleeding that requires emergent intervention, and postmenopausal uterine bleeding are also abnormal, but are beyond the scope of this article.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Adolescents with excessive uterine bleeding should be evaluated for bleeding disorders, such as von Willebrand disease. | C | 4, 6, 15 | Consensus guidelines |
| Saline infusion sonohysterography is more sensitive and specific for the detection of endometrial abnormalities than transvaginal ultrasonography. | C | 21, 22 | Meta-analysis and a small prospective comparison trial |
| The levonorgestrel-releasing intrauterine system (Mirena) is an effective treatment for menorrhagia, with patient satisfaction scores similar to endometrial ablation and hysterectomy. | A | 35, 44 | Cochrane review and randomized trial |
| NSAIDs are effective in reducing heavy menstrual blood flow. There is no evidence that one NSAID is more effective than another. | B | 36 | Cochrane review of nine small randomized controlled trials |
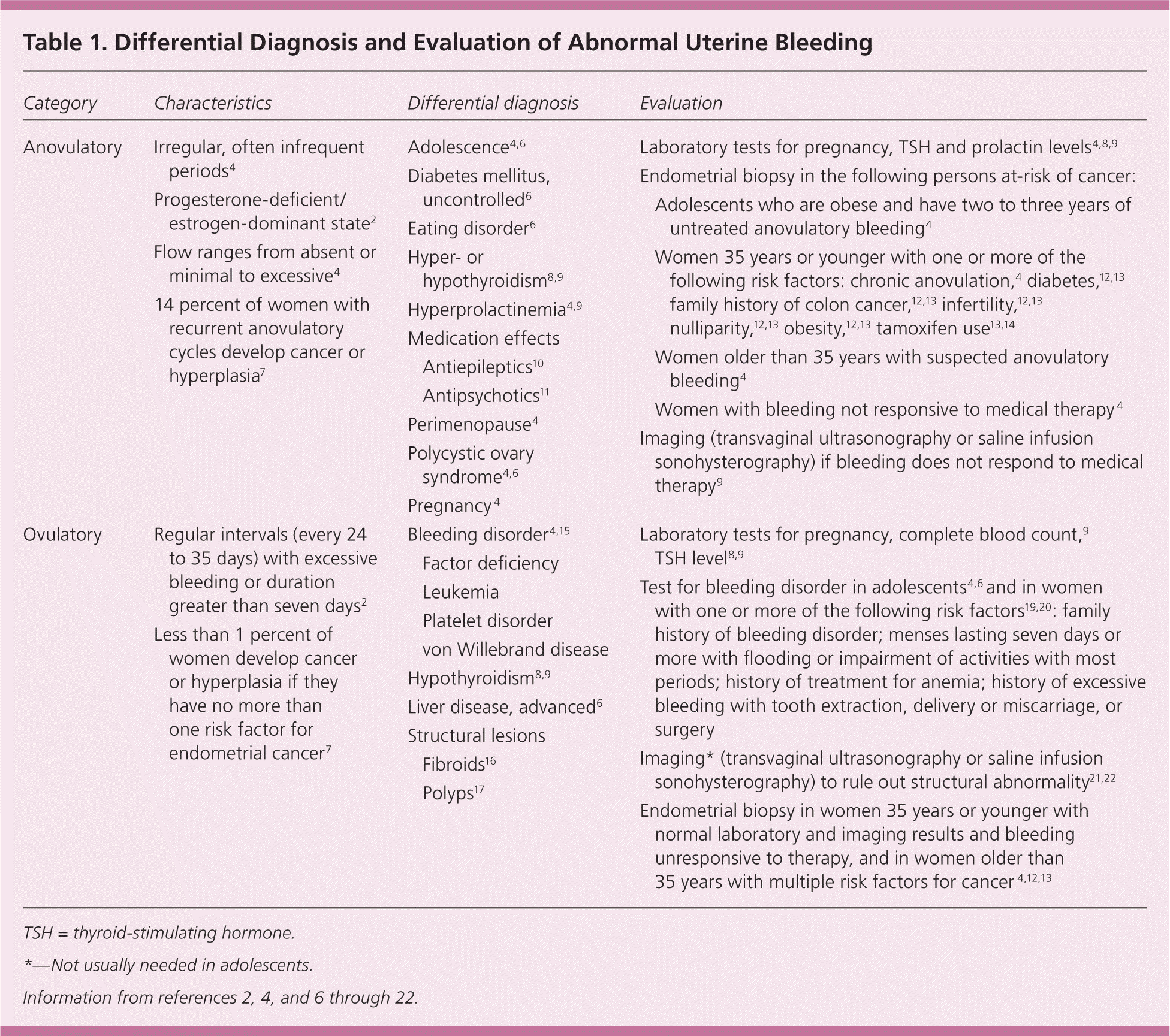
Terms associated with abnormal uterine bleeding are inconsistently defined in the literature, complicating the approach to evaluation and management.3 International experts are working to develop consensus on these definitions to improve evidence-based care.3 Abnormal uterine bleeding that occurs from adolescence through perimenopause can be broadly divided into two categories: anovulatory and ovulatory. Anovulatory bleeding is characterized by irregular or infrequent periods, with flow ranging from light to excessively heavy.4 Terms commonly associated with anovulatory bleeding include amenorrhea (absence of periods for more than three cycles), oligomenorrhea (menses occurring at intervals of more than 35 days), metrorrhagia (menses at irregular intervals with excessive bleeding or lasting more than seven days), and dysfunctional uterine bleeding (anovulatory bleeding in which underlying etiologies have been ruled out).2 The term dysfunctional uterine bleeding is sometimes used to encompass many other abnormal uterine bleeding patterns, so for clarity and consistency with the American College of Obstetricians and Gynecologists (ACOG), this article uses the term anovulatory abnormal uterine bleeding.4
In contrast to anovulatory patterns, ovulatory abnormal uterine bleeding (menorrhagia) occurs at regular intervals (every 24 to 35 days), but with excessive volume or duration of more than seven days.2 Excessive menstrual bleeding is defined as the need to change menstrual products every one to two hours, passage of clots greater than 1 inch (2.54 cm), and/or “very heavy” periods as reported by the patient.5,6 It is commonly associated with low ferritin levels.5 Table 1 summarizes the characteristics, differential diagnosis, and evaluation of anovulatory and ovulatory abnormal uterine bleeding.2,4,6–22
| Category | Characteristics | Differential diagnosis | Evaluation | |||
|---|---|---|---|---|---|---|
| Anovulatory |
| |||||
| Ovulatory |
| |||||
Anovulatory Bleeding
At extremes of the reproductive years, irregular cycles resulting from anovulation can occur. Following menarche, the immature hypothalamic-pituitary-ovarian axis may result in anovulatory cycles for two to three years.2,6 Up to eight years before menopause, women may again have intermittent anovulatory cycles.2 During the rest of the reproductive years, however, recurrent irregular cycles may be caused by anovulation and are considered abnormal.4
When ovulation does not occur, no corpus luteum forms to produce progesterone, leading to prolonged estrogenic stimulation of the endometrium, excessive proliferation, endometrial instability, and erratic bleeding.4 Approximately 6 to 10 percent of women with anovulation have underlying polycystic ovary syndrome.4 Uncontrolled diabetes mellitus,6 hypo- or hyperthyroidism,8,9 and hyperprolactinemia4 also may cause anovulation by interfering with the hypothalamic-pituitary-ovarian axis.6 Antiepileptics (especially valproic acid [Depakene]) may cause weight gain, hyperandrogenism, and anovulation.10 Use of typical antipsychotics (e.g., haloperidol, chlorpromazine, thiothixene [Navane]) and some atypical antipsychotics (e.g., clozapine [Clozaril], risperidone [Risperdal]) may contribute to anovulation by raising prolactin levels.11
Recurrent anovulation causes an increased risk of endometrial cancer.4,7,12 Endometrial carcinoma in adolescents is rare, but has been reported and should be considered if recurrent anovulation for two to three years or morbid obesity is present.23 About 14 percent of premenopausal women with recurrent anovulatory cycles develop endometrial cancer or its precursor, hyperplasia with atypia.7 Ten to 20 percent of endometrial cancers are diagnosed in premenopausal women.13,14 Women at highest risk of cancer have advanced age, obesity, nulliparity, infertility, diabetes, family history of colon cancer, long-term unopposed estrogen therapy, or a history of tamoxifen use.4,12–14 One study demonstrated the highest incidence of endometrial abnormality, ranging from hyperplasia without atypia to cancer, in premenopausal women 45 years or older with abnormal uterine bleeding (number needed to screen [NNS] = 13), women weighing 198 lb (90 kg) or greater (NNS = 8), or both (NNS = 5).13 Hyperplasia without atypia is generally considered benign, with less than 5 percent of cases progressing to cancer.24,25 In contrast, 30 percent of cases of hyperplasia with atypia progress to cancer,24 and 42.6 percent of women with this pathology have undiagnosed, concurrent endometrial adenocarcinoma.26
EVALUATION
Patients with irregular cycles who should be evaluated include adolescents with consistently more than three months between cycles6 or those with irregular cycles for more than three years4; women with suspected recurrent anovulatory cycles4; and women who are likely perimenopausal and have increased volume or duration of bleeding over baseline, periods more often than every 21 days, intermenstrual spotting, or postcoital bleeding.27
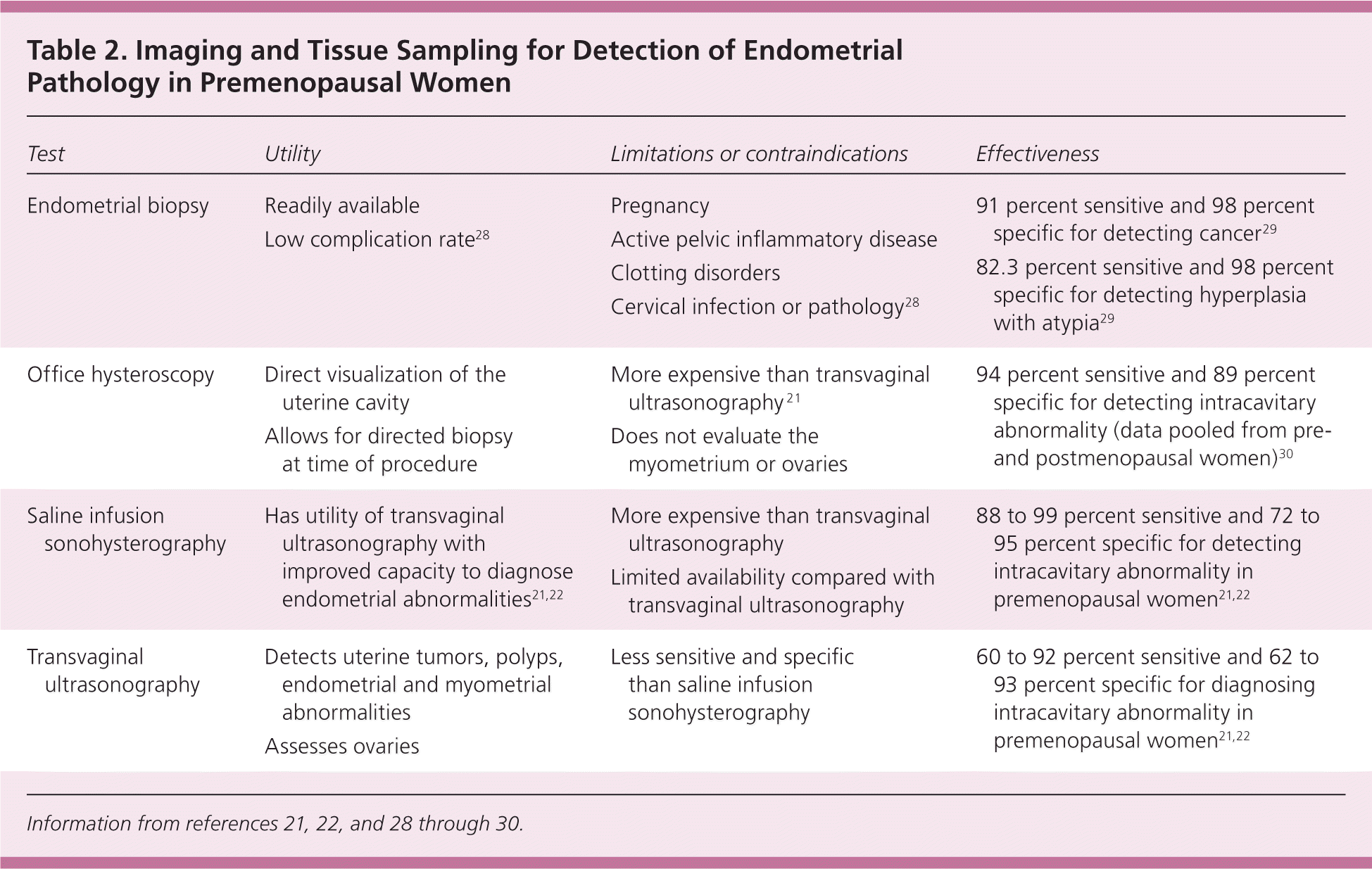
Initial evaluation of anovulatory uterine bleeding should include history, physical examination to look for obesity and hirsutism (manifestations of polycystic ovary syndrome),4,6 a pregnancy test, and measurement of thyroid-stimulating hormone4,8,9 and prolactin levels.4,9 ACOG recommends endometrial tissue assessment to rule out cancer in adolescents and in women younger than 35 years with prolonged unopposed estrogen stimulation, women 35 years or older with suspected anovulatory bleeding, and women unresponsive to medical therapy.4 Office endometrial biopsy is relatively inexpensive, convenient, and has a low risk of complications.28 Findings may include benign endometrium, simple or complex hyperplasia without atypia, hyperplasia with atypia, or endometrial adenocarcinoma.2,14 In premenopausal women, endometrial biopsy is 82.3 percent sensitive for detecting hyperplasia with atypia and 91 percent sensitive for detecting endometrial cancer; specificity is 98 percent for both29 (Table 221,22,28–30 ).
| Test | Utility | Limitations or contraindications | Effectiveness |
|---|---|---|---|
| Endometrial biopsy |
|
| 91 percent sensitive and 98 percent specific for detecting cancer29 |
| 82.3 percent sensitive and 98 percent specific for detecting hyperplasia with atypia29 | |||
| Office hysteroscopy |
|
| 94 percent sensitive and 89 percent specific for detecting intracavitary abnormality (data pooled from pre- and postmenopausal women)30 |
| Saline infusion sonohysterography |
| 88 to 99 percent sensitive and 72 to 95 percent specific for detecting intracavitary abnormality in premenopausal women21,22 | |
| Transvaginal ultrasonography |
|
| 60 to 92 percent sensitive and 62 to 93 percent specific for diagnosing intracavitary abnormality in premenopausal women21,22 |
Women at low risk of endometrial cancer and women with benign endometrial histology who have continued irregular or excessive uterine bleeding despite treatment should undergo imaging to rule out concomitant structural changes.4,9 If no abnormalities are found, hysteroscopy should be considered.4 Figure 1 is an algorithm for the evaluation and treatment of anovulatory abnormal uterine bleeding.4,8,9,12–14,31
TREATMENT
There is little consensus on specific treatment regimens for anovulatory uterine bleeding.32 Pharmacologic treatment options are listed in Table 3.4,9,11,14,31,33–39 ACOG recommends treatment with combination oral contraceptives or cyclic progestin.4 Progestin therapy and oral contraceptives induce routine withdrawal bleeding, decrease the risk of hyperplasia or cancer, and correct any related excessive menstrual bleeding.4 Oral contraceptives containing 35 mcg or less of ethinyl estradiol are preferred.4 Cyclic oral medroxyprogesterone acetate (Provera) at a dosage of 10 mg per day for 10 to 14 days per month also is effective.9
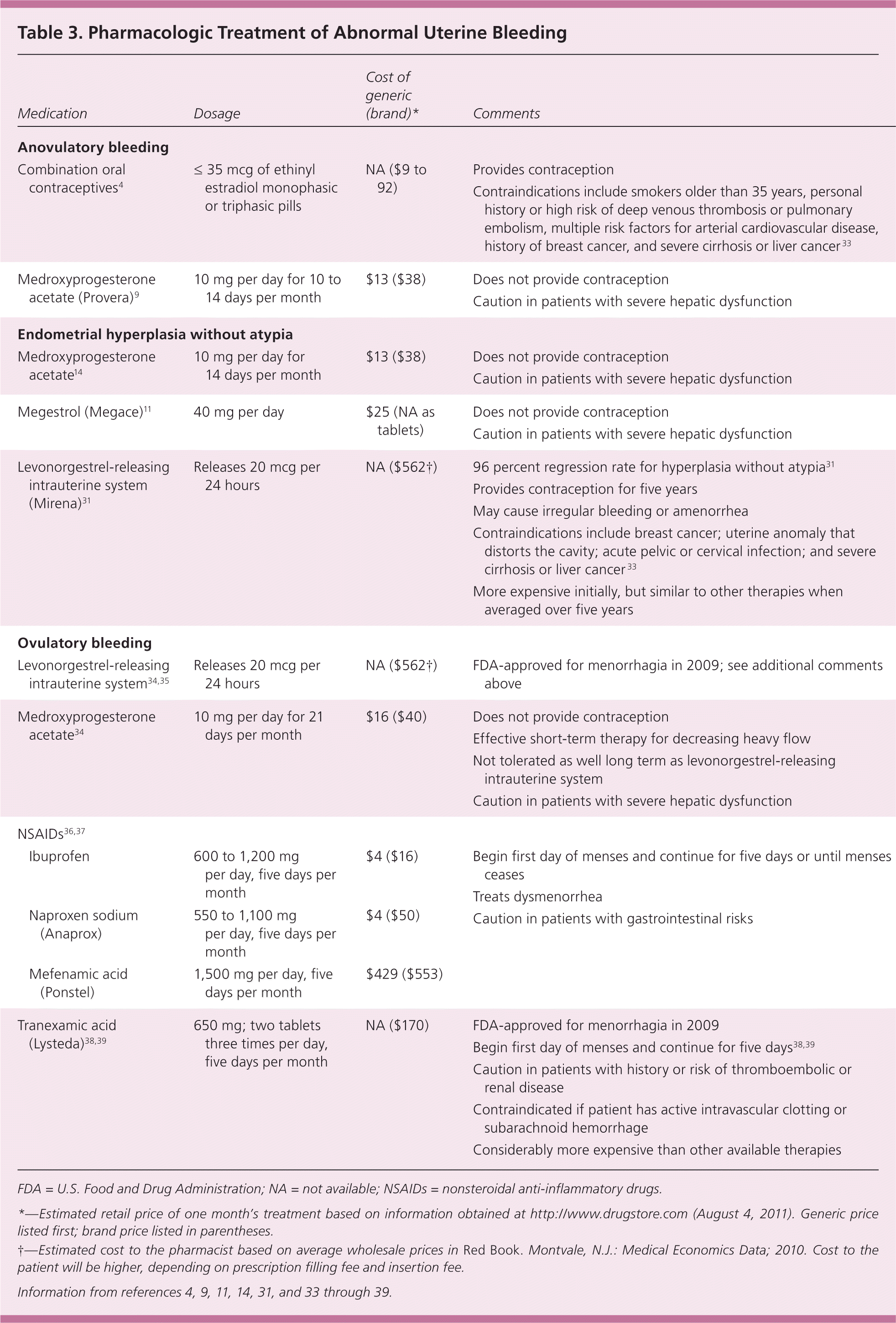
| Medication | Dosage | Cost of generic (brand)* | Comments | |
|---|---|---|---|---|
| Anovulatory bleeding | ||||
| Combination oral contraceptives4 | ≤ 35 mcg of ethinyl estradiol monophasic or triphasic pills | NA ($9 to 92) | Provides contraception | |
| Contraindications include smokers older than 35 years, personal history or high risk of deep venous thrombosis or pulmonary embolism, multiple risk factors for arterial cardiovascular disease, history of breast cancer, and severe cirrhosis or liver cancer33 | ||||
| Medroxyprogesterone acetate (Provera)9 | 10 mg per day for 10 to 14 days per month | $13 ($38) | Does not provide contraception | |
| Caution in patients with severe hepatic dysfunction | ||||
| Endometrial hyperplasia without atypia | ||||
| Medroxyprogesterone acetate14 | 10 mg per day for 14 days per month | $13 ($38) | Does not provide contraception | |
| Caution in patients with severe hepatic dysfunction | ||||
| Megestrol (Megace)11 | 40 mg per day | $25 (NA as tablets) | Does not provide contraception | |
| Caution in patients with severe hepatic dysfunction | ||||
| Levonorgestrel-releasing intrauterine system (Mirena)31 | Releases 20 mcg per 24 hours | NA ($562†) | 96 percent regression rate for hyperplasia without atypia31 | |
| Provides contraception for five years | ||||
| May cause irregular bleeding or amenorrhea | ||||
| Contraindications include breast cancer; uterine anomaly that distorts the cavity; acute pelvic or cervical infection; and severe cirrhosis or liver cancer33 | ||||
| More expensive initially, but similar to other therapies when averaged over five years | ||||
| Ovulatory bleeding | ||||
| Levonorgestrel-releasing intrauterine system34,35 | Releases 20 mcg per 24 hours | NA ($562†) | FDA-approved for menorrhagia in 2009; see additional comments above | |
| Medroxyprogesterone acetate34 | 10 mg per day for 21 days per month | $16 ($40) | Does not provide contraception | |
| Effective short-term therapy for decreasing heavy flow | ||||
| Not tolerated as well long term as levonorgestrel-releasing intrauterine system | ||||
| Caution in patients with severe hepatic dysfunction | ||||
| NSAIDs36,37 | ||||
| Ibuprofen | 600 to 1,200 mg per day, five days per month | $4 ($16) | Begin first day of menses and continue for five days or until menses ceases | |
| Treats dysmenorrhea | ||||
| Caution in patients with gastrointestinal risks | ||||
| Naproxen sodium (Anaprox) | 550 to 1,100 mg per day, five days per month | $4 ($50) | Begin first day of menses and continue for five days or until menses ceases | |
| Treats dysmenorrhea | ||||
| Caution in patients with gastrointestinal risks | ||||
| Mefenamic acid (Ponstel) | 1,500 mg per day, five days per month | $429 ($553) | Begin first day of menses and continue for five days or until menses ceases | |
| Treats dysmenorrhea | ||||
| Caution in patients with gastrointestinal risks | ||||
| Tranexamic acid (Lysteda)38,39 | 650 mg; two tablets three times per day, five days per month | NA ($170) | FDA-approved for menorrhagia in 2009 | |
| Begin first day of menses and continue for five days38,39 | ||||
| Caution in patients with history or risk of thromboembolic or renal disease | ||||
| Contraindicated if patient has active intravascular clotting or subarachnoid hemorrhage | ||||
| Considerably more expensive than other available therapies | ||||
Treatment options for women who have hyperplasia without atypia include cyclic medroxyprogesterone acetate at 10 mg per day for 14 days per month, continuous megestrol (Megace) at 40 mg per day,14 or the levonorgestrel-releasing intrauterine system (Mirena).31 After the initiation of treatment, endometrial biopsy should be repeated in three to six months to assure resolution of the hyperplasia.9,14 Because of the high rate of progression to cancer, women found to have hyperplasia with atypia should be referred to a gynecologist to review treatment options.14 Hysterectomy is the recommended treatment, but women desiring continued fertility may be candidates for progestin therapy and close follow-up.14 Women found to have adenocarcinoma should be referred to a gynecologic oncologist for hysterectomy and staging.14
Ovulatory Bleeding
Ovulatory abnormal uterine bleeding, or menorrhagia, presents as bleeding that occurs at normal, regular intervals but that is excessive in volume or duration.2 Hypothyroidism,8,9 late-stage liver disease,6 or bleeding disorders4,6 may cause menorrhagia, as may structural changes, such as submucosal fibroids or endometrial polyps.16,17 Von Willebrand disease (vWD), the most common heritable bleeding disorder,19 is present in approximately 13 percent of women with menorrhagia.18 The prevalence is likely higher in adolescents presenting with excessive uterine bleeding.6,15 In contrast to women with anovulatory bleeding, women with ovulatory bleeding produce progesterone, slough the endometrium regularly, and have minimal risk of developing cancer.7 Approximately one-half of women with menorrhagia have no discernable cause.40
EVALUATION
Initial evaluation of menorrhagia should include a pregnancy test, complete blood count,9 and measurement of thyroid-stimulating hormone level.8,9 The American Academy of Pediatrics and ACOG recommend evaluating adolescents with menorrhagia for possible bleeding disorders, specifically vWD.6,15 A woman with menorrhagia should be evaluated for a possible bleeding disorder if she has one or more of the following: a family history of bleeding disorder; menses lasting seven days or more with flooding or impairment of activities with most periods; a history of treatment for anemia; or a history of excessive bleeding with tooth extraction, delivery or miscarriage, or surgery.20 Initial testing for bleeding disorders includes complete blood count (to assess for anemia, leukemia, and thrombocytopenia), and prothrombin and activated partial thromboplastin time (to assess for factor deficiencies).6,19 The approach to further testing for bleeding disorders, specifically vWD, is varied, and collaboration with a hematologist is recommended.6,15,19,20
Although uncommon in adolescents,41 uterine polyps and fibroids may underlie menorrhagia in women.16,17 Transvaginal ultrasonography is used to evaluate the ovaries, uterus, and endometrium.21,22 Saline infusion sonohysterography is the intrauterine infusion of saline during transvaginal ultrasonography that provides enhanced views of the endometrium.21 In two small studies of premenopausal women, transvaginal ultrasonography had 60 to 92 percent sensitivity and 62 to 93 percent specificity for diagnosing intracavitary lesions. In both studies, saline infusion sonohysterography improved sensitivity to 88 to 99 percent and specificity to 72 to 95 percent.21,22
If no etiology is found on ultrasonography, if bleeding is unresponsive to medical therapy, or if there are considerable risks of endometrial cancer, additional evaluation with endometrial biopsy4 or direct visualization of the endometrium with hysteroscopy is recommended.30 Hysteroscopy has a 94 percent sensitivity and 89 percent specificity for detecting intracavitary abnormalities.30
TREATMENT
The goals of treatment for menorrhagia are to reduce flow volume and to correct anemia. Hormonal and non-hormonal therapeutic options are available to patients (Table 34,9,11,14,31,33–39 ). Figure 2 is an algorithm for the evaluation and treatment of ovulatory abnormal uterine bleeding.2,4,6,8,9,15–22,30,34–39,42,43
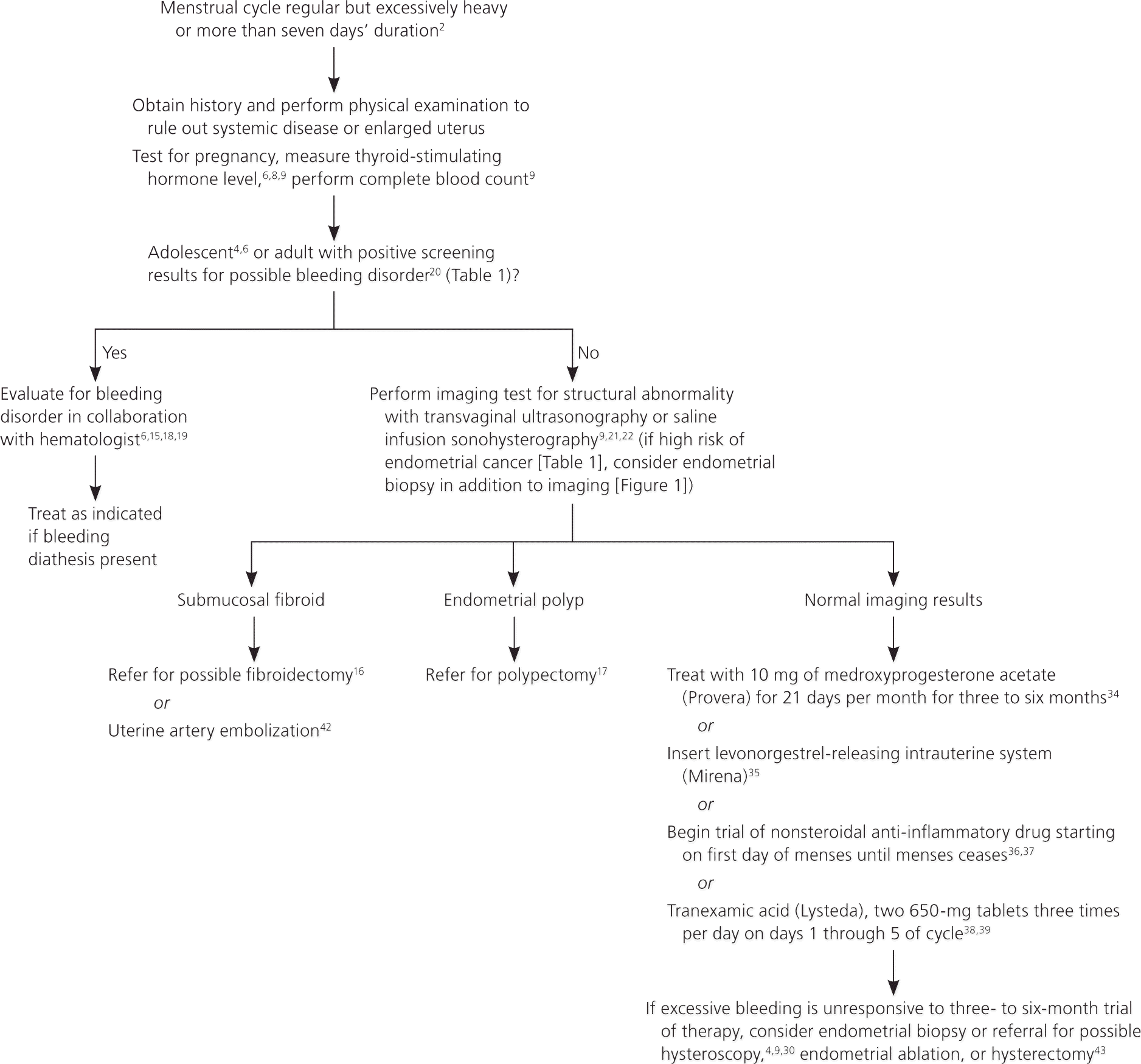
Hormonal Therapies. Progestins effectively decrease excessive menstrual bleeding. In contrast to the shorter course of oral progestin therapy used for anovulatory uterine bleeding, progestin therapy for menorrhagia needs to be given for 21 days per month to be effective.34 The continuous progesterone release provided by the levonorgestrel-releasing intrauterine system reduces menorrhagia more effectively than oral progestins.35 It is better tolerated than the 21-day oral regimen and has patient satisfaction scores similar to endometrial ablation and hysterectomy at a significantly lower cost.35,44 The levonorgestrel-releasing intrauterine system is the only contraceptive approved by the U.S. Food and Drug Administration (FDA) for the treatment of menorrhagia.
Oral contraceptives have been shown to reduce menstrual flow volume, especially when used continuously,45 but they have not been studied specifically in women with menorrhagia. Consequently, there are few data to support their effectiveness.46 Oral contraceptives are, however, the treatment of choice in women with known vWD who also desire contraception.19
Nonhormonal Therapies. At scheduled pharmacologic doses, nonsteroidal anti-inflammatory drugs (NSAIDs) decrease prostaglandin levels, reducing menstrual bleeding.36 In one small study, naproxen sodium (Anaprox) and mefenamic acid (Ponstel) decreased flow volume by 46 and 47 percent, respectively.37 There is no evidence that one NSAID is more effective than another,36 but cost varies considerably.
Tranexamic acid (Lysteda), an antifibrinolytic that prevents activation of plasminogen, is FDA-approved for the treatment of menorrhagia. Two 650-mg tablets taken three times per day for the first five days of the cycle decreased bleeding significantly more than NSAIDs did.38 Although increased rates of thrombosis were initially a concern, long-term studies have not demonstrated this.38 Cost remains a limiting factor of tranexamic acid. It is likely most appropriate in women with bleeding disorders who desire fertility or have contraindications to oral contraceptives.
Surgery. Uterine polyps and leiomyomas, specifically submucosal fibroids, may cause menorrhagia. Available evidence suggests that hysteroscopic polypectomy reduces 75 to 100 percent of abnormal uterine bleeding symptoms in women with endometrial polyps.17 For menorrhagia associated with submucosal fibroids, surgical resection may allow women to maintain child-bearing capacity.16 Resection may normalize menses, but the clear long-term impact on reproduction is unknown.16 Alternatively, fibroids may be treated with uterine artery embolization, the percutaneous embolization of perifibroid vessels causing infarction of the fibroid.42 The effect of uterine artery embolization on future pregnancies also needs further study.42 Whether abnormal uterine bleeding caused by fibroids is treated with surgical resection or uterine artery embolization, approximately 20 percent of women subsequently undergo a hysterectomy for recurrent abnormal uterine bleeding.16,42
If excessive uterine bleeding is unresponsive to medical intervention, endometrial ablation (the surgical destruction of the endometrium) may be considered.43 This intervention is considered permanent and not advised in women who desire continued fertility. By five years postablation, approximately one-third of women require a second operation.43
Hysterectomy is the definitive treatment for excessive uterine bleeding in women who no longer wish to conceive. Disadvantages include increased number of adverse effects, longer recovery time, and higher initial health care costs compared with uterine-sparing procedures.42,43 Hysterectomy also may be associated with ovarian failure nearly four years earlier than expected.47