
A more recent article on diagnosis and treatment of melanoma is available.
Am Fam Physician. 2012;85(2):161-168
Patient information: See related handout on cutaneous malignant melanoma, written by the author of this article.
Author disclosure: No relevant financial affiliations to disclose.
Cutaneous malignant melanoma accounts for 3 to 5 percent of all skin cancers and is responsible for approximately 75 percent of all deaths from skin cancer. Persons with an increased number of moles, dysplastic (also called atypical) nevi, or a family history of the disease are at increased risk compared with the general population. An important tool to assist in the evaluation of potential melanomas for patients and health care professionals is the ABCDE mnemonic, which takes into account asymmetry, border irregularities, color variation, diameter, and evolution. Any suspicious pigmented lesion should be biopsied. Appropriate methods of biopsy can vary, and include deep shave, punch, and excisional biopsy. Regardless of the procedure selected, it is essential that the size of the specimen be adequate to determine the histologic depth of lesion penetration, which is known as the Breslow depth. The Breslow depth is the most important prognostic parameter in evaluating the primary tumor. Because early detection and treatment can lead to identification of thinner lesions, which may increase survival, it is critical that physicians be comfortable with evaluating suspicious pigmented lesions and providing treatment or referral as necessary.
Cutaneous malignant melanoma (CMM) is a potentially lethal form of skin cancer. Although it comprises only 3 to 5 percent of all skin cancers, it is responsible for approximately 75 percent of all deaths from skin cancers.1,2 CMM results from the malignant transformation of melanocytes, which are the pigment-producing cells responsible for the color of skin. The key triggers leading to malignant transformation of melanocytes have yet to be fully elucidated, but are multifactorial and include UV radiation damage and genetic susceptibility.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Some organizations recommend including a skin examination during periodic health examinations for adults; however, the U.S. Preventive Services Task Force found insufficient evidence to recommend for or against annual screening for skin cancer. | C | 2, 8, 9 | — |
| There is no statistically significant difference in survival for narrow vs. wide surgical margins for treatment of cutaneous malignant melanoma. | B | 1 | Statistically insignificant benefit for wide margins |
| Sentinel node biopsy in persons with melanoma with a Breslow depth of 1.0 mm or greater is useful for determining staging and prognosis. | C | 20, 30, 32 | Sentinel node biopsy is effective for determining staging and prognosis; no increase in survival has been proven |
Incidence and Mortality
The incidence of CMM is highest in white persons. This population is approximately 20 times more likely to develop the disease than black persons. It is rare in young persons, with only 2 percent of melanomas occurring in persons younger than 20 years and approximately 0.3 percent in children younger than 14 years.3 It is the most common cancer in women 25 to 29 years of age, and is second only to breast cancer in women 30 to 34 years of age.4 Based on 2004 to 2008 data, the overall age-adjusted incidence of CMM was 20.8 per 100,000 men and women per year (Table 1).5 However, the annual incidence has increased significantly in white men older than 65 years (8.8 percent per year since 2003), and these men are twice as likely as women in the same age group to develop CMM.4,6 Persons with an increased number of moles, dysplastic (also called atypical) nevi, or a family history of the disease are at increased risk compared with the general population.
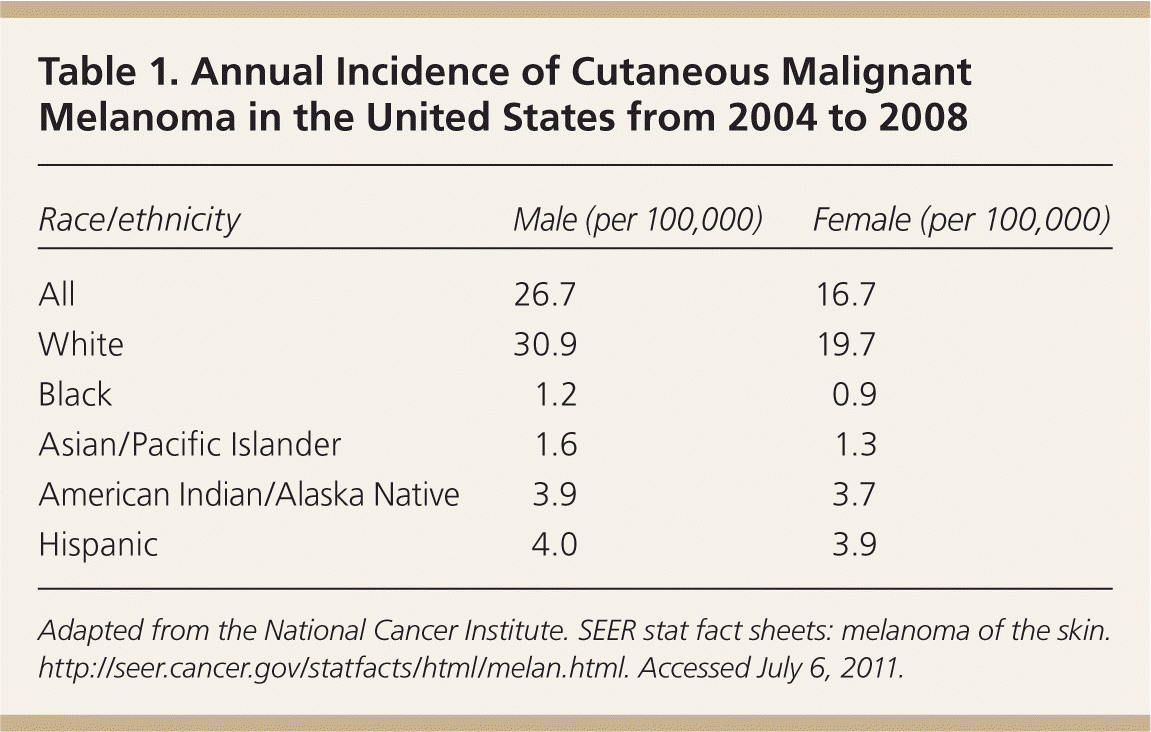
| Race/ethnicity | Male (per 100,000) | Female (per 100,000) |
|---|---|---|
| All | 26.7 | 16.7 |
| White | 30.9 | 19.7 |
| Black | 1.2 | 0.9 |
| Asian/Pacific Islander | 1.6 | 1.3 |
| American Indian/Alaska Native | 3.9 | 3.7 |
| Hispanic | 4.0 | 3.9 |
Diagnosis
CMM can occur de novo or in a preexisting nevus; therefore, close examination of all nevi on a patient is necessary.7 This may be a daunting task in a busy primary care environment, particularly given the other myriad problems that require evaluation at any particular visit. Some organizations recommend including a skin examination during periodic health examinations for adults; however, the U.S. Preventive Services Task Force concluded that there is not enough evidence to recommend for or against annual whole-body skin examination by a primary care physician or patient for early detection of skin cancers in the adult general population.2,8,9 This recommendation was directed toward patients without a history of premalignant or malignant lesions. Although it makes intuitive sense to counsel patients on disease prevention and to screen them periodically, there is not yet strong evidence to support these practices. The Web site FamilyDoctor.org presents a guideline for patients on skin cancer prevention and self-screening at https://familydoctor.org/familydoctor/en/diseases-conditions/skin-cancer.html.
ABCDE MNEMONIC
The ABCD mnemonic for pigmented skin lesions was first devised in 1985, but continues to be an important tool for patients and health care professionals.10–14 It was further modified in 2004 with the addition of “E” to identify evolving lesions. The mnemonic takes into account asymmetry, border irregularities, color variation, diameter, and evolution to assist in the evaluation of potential melanomas (Table 215 ). Evolution of a lesion has been shown in some studies to be the most specific finding that may indicate melanoma.16,17
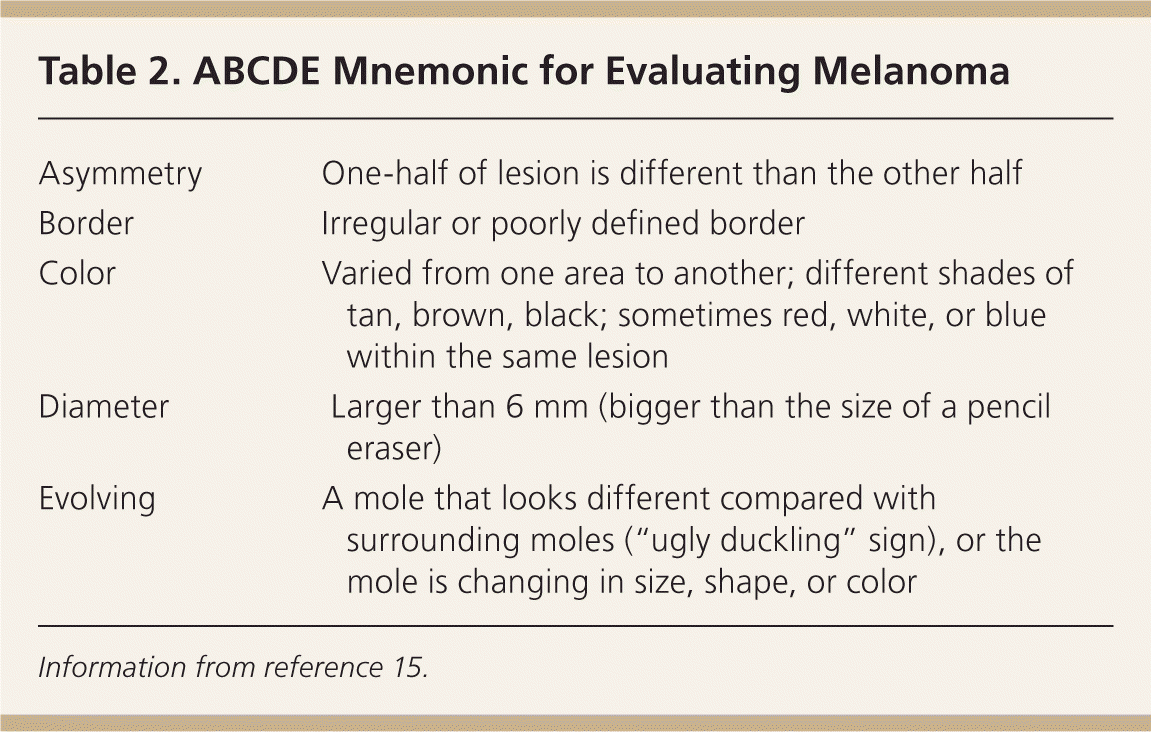
| Asymmetry | One-half of lesion is different than the other half |
| Border | Irregular or poorly defined border |
| Color | Varied from one area to another; different shades of tan, brown, black; sometimes red, white, or blue within the same lesion |
| Diameter | Larger than 6 mm (bigger than the size of a pencil eraser) |
| Evolving | A mole that looks different compared with surrounding moles (“ugly duckling” sign), or the mole is changing in size, shape, or color |
“UGLY DUCKLING” SIGN
The “ugly duckling” sign is a recent and novel technique to aid in the detection of melanoma. It is a simple and easy-to-teach method based on the concept that a melanoma may look different compared with surrounding moles.18
DERMOSCOPY
Dermoscopy (also termed dermatoscopy in some literature) is used in the evaluation of many types of skin lesions. A device (called a dermascope or dermatoscope) shines polarized light on the skin and magnifies skin lesions with or without a fluid interface (i.e., water, immersion oil, or alcohol), allowing the physician to see pigment and structures in the skin without obstruction by skin surface reflections. Pigment and structures in the skin can give important clues to histology. CMM can have some highly specific dermoscopic features, which can increase the index of suspicion regarding a given lesion and lead the primary care physician to perform a biopsy.
Dermoscopy is a useful tool that has been shown to increase the accuracy of a dermatologist in diagnosing a melanoma by 10 to 27 percent.16 In one study of primary care physicians who used dermoscopy after a one-day training course, the physicians were able to increase their sensitivity for detecting melanoma by approximately 25 percent.16 Training is paramount to the success of dermoscopy. Continuing medical education courses and online tutorials are available. A series of videos produced by Thomas Habif, MD, is useful and includes information on dermoscopy (http://www.youtube.com/watch?v=GkP_Jf6utR4); dermascopes (http://www.youtube.com/watch?v=29Rv2EcBTMs); superficial spreading melanoma (http://www.youtube.com/watch?v=JMIkd6devDo); and color of nevi (http://www.youtube.com/watch?v=V8Z6XDcz53g).
BIOPSY
Any suspicious pigmented lesion should be biopsied. However, the actual method performed has been the source of much study. The generally preferred method is complete elliptical excision of the suspicious lesion with a small margin of normal-appearing skin (approximately 3 mm). It is important to clearly document the dimensions of the lesion in the physician's procedure note, with the dimensions of the lesion plus the surgical margin (e.g., preoperative size: 3 × 4 mm; surgical margins: 3 mm; postoperative size: 9 × 10 mm; postoperative wound length: “X” mm or cm). This will serve as a guide for what additional surgical margins may be necessary if a melanoma is diagnosed.
A scoop shave biopsy (saucerization) and a punch biopsy can be appropriate options depending on the comfort level of the physician and the clinical situation.19–21 Given that the Breslow depth of CMM is crucial to staging and prognosis, a superficial shave of a suspicious pigmented lesion should be avoided.20 If a melanoma is bisected during biopsy in such a way that the depth of the lesion cannot be determined, this crucial staging tool will be lost. In the case of smaller pigmented lesions, if excisional biopsy is not practical and the entire lesion can be removed with punch biopsy, then punch biopsy is an appropriate option. However, with a larger lesion (particularly a suspected lentigo maligna melanoma), a single punch may not capture the malignant portion,16 and it may be necessary to perform a larger deep shave biopsy of the portion of the lesion with the most atypical appearing pigmentation network. The most important aspect of any method is to obtain an adequate specimen that will allow for a definitive diagnosis (including the measurement of the Breslow depth) or exclusion of melanoma by an experienced pathologist or dermatopathologist.
Proper staging of a melanoma is based on a combination of histologic and clinical features.20 Breslow depth, ulceration, and mitotic rate (number of mitoses per square millimeter) are three crucial features in the staging and ultimate prognosis of a patient's disease. Although presence of tumor ulceration increases the stage of a specific tumor, the Breslow depth and mitotic rate are the most powerful predictors of survival.20
TYPES
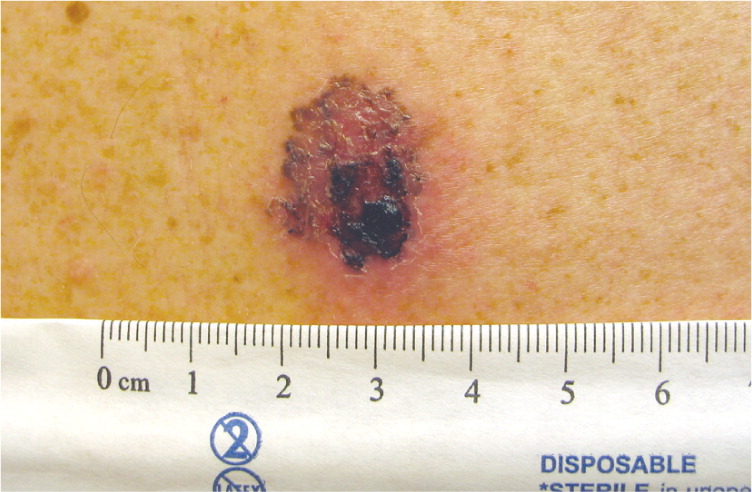
Superficial Spreading Melanoma (Figure 2). This is the most common type of CMM in persons with fair skin. It often has an initial slow radial growth phase before becoming invasive. Superficial spreading melanoma often begins as an asymptomatic brown or black spot (macule) that may have asymmetry, irregular borders, or variations in color.7
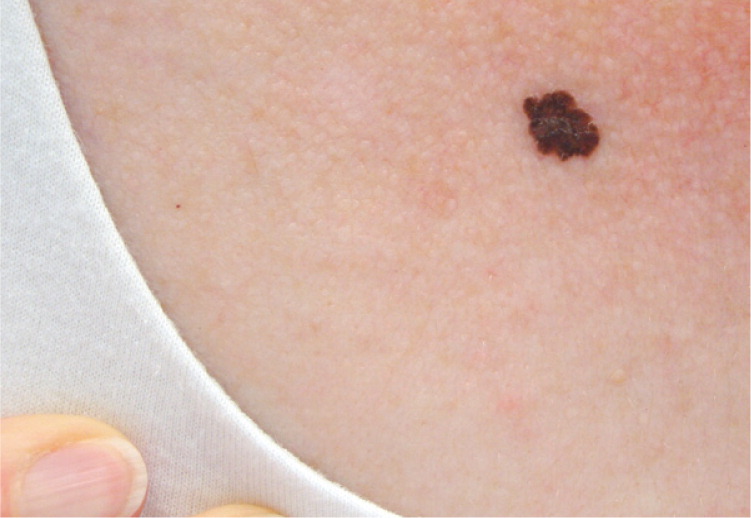
Nodular Melanoma (Figure 3). This is the second most common type of CMM in persons with fair skin. It is not believed to have the radial growth phase that occurs with superficial spreading melanoma.22 Nodular melanoma progresses rapidly as a vertically growing tumor over months, and typically presents as a blue to black nodule, although it can sometimes be pink or red in color. These lesions also may have ulceration and bleeding.
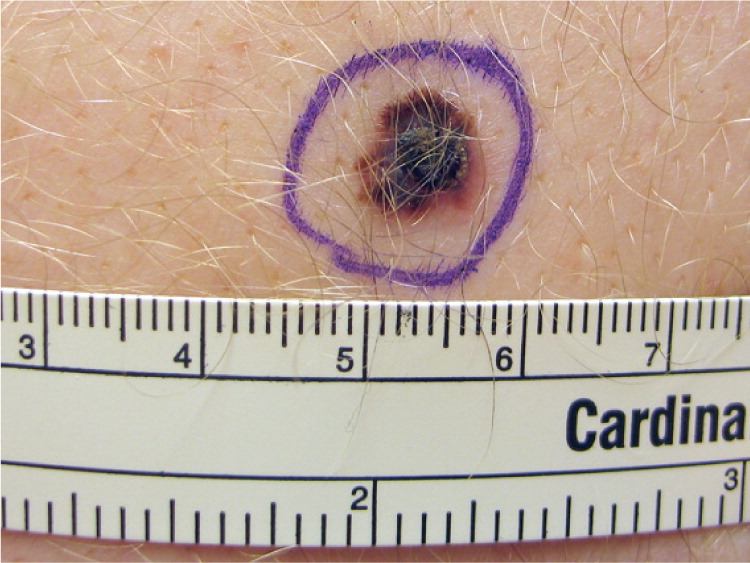
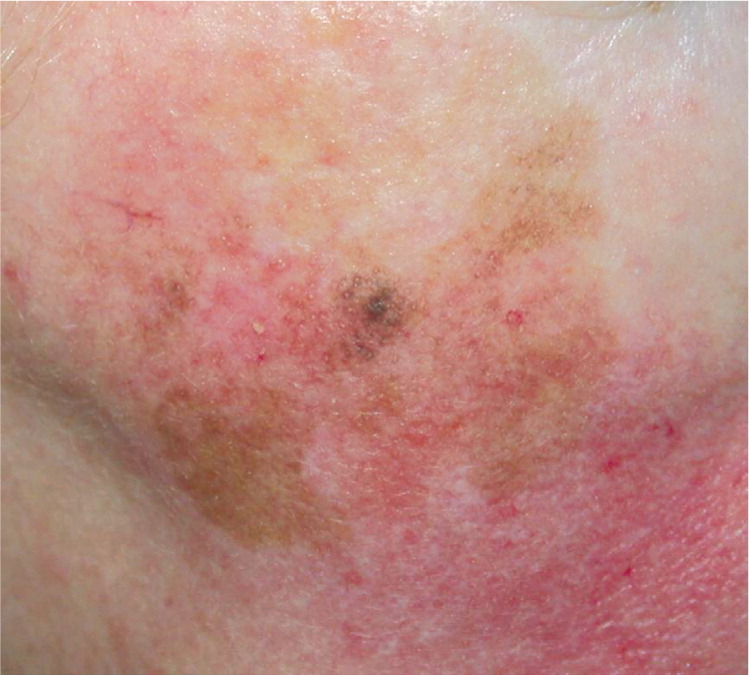
Lentigo Maligna and Lentigo Maligna Melanoma (Figure 423 ). These lesions most often occur in persons 60 to 80 years of age with heavily sun-damaged skin.22 Lentigo maligna is an in situ form that slowly grows over five to 15 years before becoming invasive (lentigo maligna melanoma). Lentigo maligna and lentigo maligna melanoma can be fairly large lesions. It is estimated that only 3 to 5 percent of lentigo maligna lesions will become invasive, and it is generally thought that the risk of progression is proportional to the size of the lesion.24 Lentigo maligna usually begins as an irregularly shaped tan spot (macule) that slowly grows to form a larger spot (patch). Invasive changes can be evident with the formation of bumps (papules), but invasion can be difficult to predict based on clinical examination. The mortality rate of lentigo maligna and lentigo maligna melanoma is the same as any other CMM, based on depth of the lesion.
Amelanotic Melanoma (Figure 5). This type of CMM is an uncommon, nonpigmented variant of melanoma (less than 5 per cent) that can mimic a wide range of benign and malignant conditions, including eczema, fungal infections, basal cell carcinoma, and squamous cell carcinoma.22 Amelanotic melanoma, although not necessarily more lethal, is more often diagnosed in a more advanced and invasive stage than the pigmented types of melanoma.22
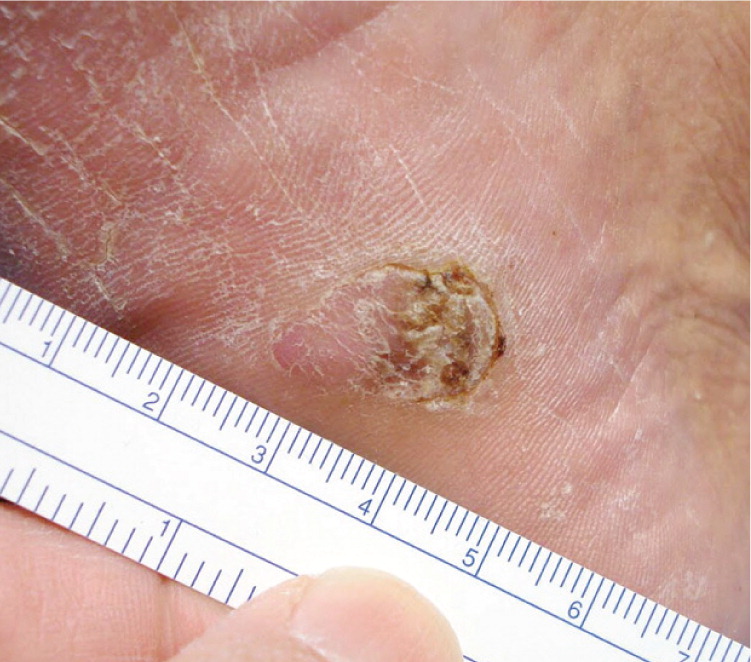
Acral-Lentiginous Melanoma (Figure 6). Acral surfaces are those found on body protrusions, such as the fingertips, knuckles, elbows, knees, buttocks, toes, heels, and ears. Specialized acral skin is found on the palms and soles and lacks hair follicles. Acral-lentiginous melanoma is the least common type of melanoma in white persons, comprising 2 to 8 percent of melanomas. However, it represents approximately 75 percent of the melanomas diagnosed in black and Asian persons.25,26 Acral-lentiginous melanoma is a major concern because of inaccurate notions that black or Asian persons cannot get CMM. As a result, it may go unnoticed until the lesion is in a more advanced stage.25,26 All patients being screened for skin cancer should have their feet examined, similar to the way that all patients with diabetes mellitus should have a foot examination.

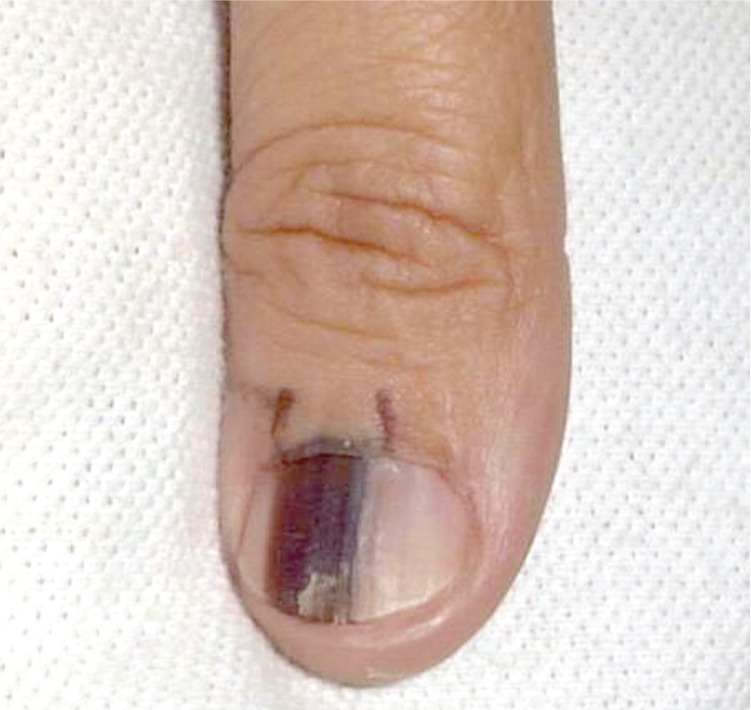
Subungual Melanoma (Figure 723 ). Melanoma of the nail arises from melanocytes in the matrix, and represents 0.7 to 3.5 percent of all melanomas.27 Although pigmented longitudinal lines on the nail plate can be normal, they can also signal that a melanoma has developed in the matrix. New-onset pigmented lines on a single nail (longitudinal melanonychia) are a concerning sign and require a biopsy into the nail matrix to properly evaluate for melanoma.28 This procedure is usually performed by a dermatologist. Patients must be counseled that such a biopsy will invariably result in permanent dystrophy of the nail.

| Type | Description | Percentage of melanomas |
|---|---|---|
| Melanoma in situ | Confined to epidermis | Approximately 40 |
| Superficial spreading melanoma | Most common type of cutaneous malignant melanoma in persons with fair skin | 70 |
| Typically occur in persons 30 to 50 years of age | ||
| Typically occur on the trunk of men and legs of women | ||
| Nodular melanoma | Second most common type of cutaneous malignant melanoma in persons with fair skin | 10 to 15 |
| Typically occur on the trunk, head, or neck | ||
| More common in men than in women | ||
| Lentigo maligna and lentigo maligna melanoma | Typically occur in persons 60 to 80 years of age with sun-damaged skin | 4 to 15 |
| Very slow growing | ||
| Can be large lesions | ||
| Amelanotic melanoma | Rare, can mimic benign conditions, often diagnosed at a more advanced stage | Less than 5 |
| Acral-lentiginous melanoma | Rare, but is the most common melanoma in black and Asian persons | 2 to 8 |
| Subungual melanoma | Rare, presents as a single longitudinal line of pigmentation on a nail | 0.7 to 3.5 |
| Pigmentation may also spread onto the overlying and adjacent proximal or lateral nail folds (Hutchinson sign) |
Surgical Treatment
Surgical removal is the primary treatment for CMM. The Breslow depth of the lesion determines definitive surgical margins (Table 4).30 The purpose of the surgical margin is to ensure complete removal of the primary lesion and any residual melanoma cells that may have spread into the surrounding skin to definitively treat the lesion and reduce the chance of recurrence. A 2009 Cochrane review involving 3,297 patients examined the association between surgical margins and survival. No statistically significant difference in survival was identified between wide (3 to 5 cm) and narrow (1 to 2 cm) surgical margins.1 This has allowed for narrower surgical margin recommendations, with less morbidity associated with the traditionally wide margins used in the past. If the physician who performed the biopsy is not providing the surgical treatment, then appropriate referral is necessary as soon as possible. Although there is no literature to identify the optimal time from diagnosis to treatment, definitive surgical treatment should usually occur no later than three to four weeks after receipt of the biopsy report.
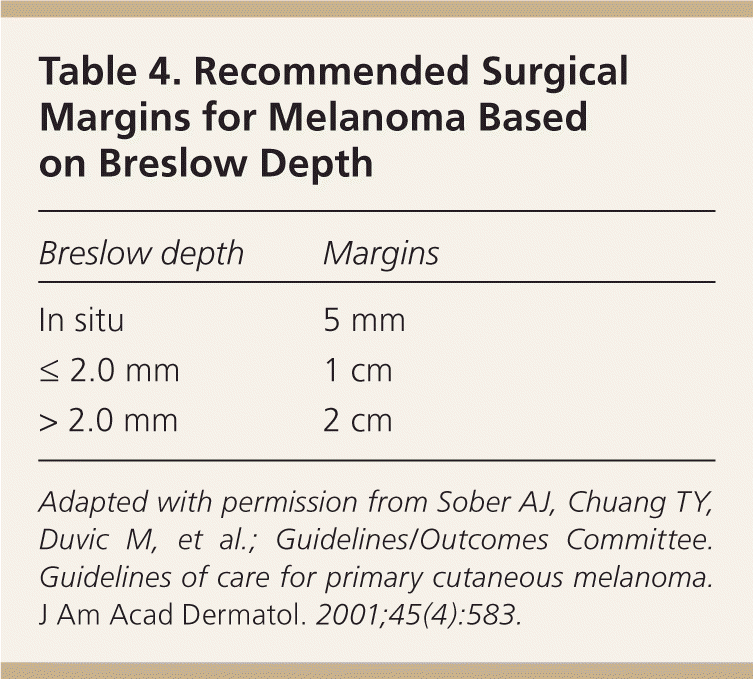
| Breslow depth | Margins |
|---|---|
| In situ | 5 mm |
| ≤ 2.0 mm | 1 cm |
| > 2.0 mm | 2 cm |
Treatment of lentigo maligna and lentigo maligna melanoma is challenging. Standard excision with 5-mm to 1-cm margins can have recurrence rates ranging from 8 to 50 percent.31 Such standard margins can leave the patient with a significant surgical defect that requires meticulous repair. Mohs surgery (also known as Mohs micrographic surgery) offers high cure rates with considerable sparing of healthy surrounding tissue. The surgery requires immunohistochemical staining of tissue (needed for identification of melanocytes) and coordination with a pathologist/dermatopathologist; however, immunohistochemical staining and access to a pathologist/dermatopathologist are not typically available in most practices that perform Mohs surgery. Although the procedure offers the possibility of better margin control, it is not yet the accepted standard of care.
In cases in which the Breslow depth is 1.0 mm or greater, the patient is often referred for a sentinel node biopsy. The sentinel node is the first node where cancer is likely to spread to from the primary tumor. Sentinel node biopsy samples the node or nodes that are thought to immediately drain the site of the lesion to identify possible nodal spread. Although the final proof regarding the effect of sentinel node biopsy on survival is yet to be universally determined, it is a useful staging tool and has less morbidity than electively removing the regional nodes.20,21,30,32
Staging and Follow-up Care
The stage of disease is determined by the TNM classification system,20 which takes into account the thickness of the tumor, degree of nodal involvement, and the extent of metastatic spread to other areas of the body. Using this staging information, a treatment plan, follow-up care plan, and statistical survival information with five- and 10-year survival estimates can be determined and discussed with the patient (Table 5).33–35
| Stage | Description | Survival (%) | Specific melanoma follow-up* | Additional testing | |
|---|---|---|---|---|---|
| 5-year | 10-year | ||||
| 0 (in situ) | Disease confined to epidermis (top layer of skin) | 99 to 100 | 99 to 100 | Every six months for first year, then every year up to year 5, then yearly for life | None |
| I | Confined to skin, but thicker (up to 1.0 mm) and has not spread | IA: 97 IB: 92 | IA: 95 IB: 86 | Every three to four months for first year, then every six months for second year, then yearly up to year 5, then yearly for life | None |
| Stage IA: skin covering melanoma remains intact | |||||
| Stage IB: skin covering melanoma may have broken open (ulcerated) | |||||
| II | Melanoma has grown thicker, ranging from 1.01 to 4.0 mm, but has not spread | IIA: 81 IIB: 70 IIC: 53 | IIA: 67 IIB: 57 IIC: 40 | Every three to four months for years 1 through 3, then every six months for years 4 and 5, then yearly for life | CBC, chemistry panel, and LDH level |
| May or may not have ulcerated | |||||
| III | Melanoma has spread to one or more nearby lymph nodes or nearby skin | IIIA: 78 IIIB: 59 IIIC: 40 | IIIA: 68 IIIB: 43 IIIC: 24 | Every three to four months for years 1 through 3, then every six months for years 4 and 5, then yearly for life | CBC, chemistry panel, and LDH level |
| PET and/or CT as determined by treating physician | |||||
| IV | Melanoma has spread to an internal organ or lymph nodes further from the original melanoma, or is found on the skin far from the original melanoma | 15 to 20 | 10 to 15 | Every three to four months for patients at high risk of relapse Follow-up in patients receiving adjuvant or palliative therapy should be based on the specific treatment prescribed | CBC, chemistry panel, and LDH level |
| PET and/or CT every two to four months for first five years and then yearly thereafter | |||||
| Additional testing in patients receiving adjuvant or palliative therapy should be based on the specific treatment prescribed | |||||
CBC = complete blood count; CT = computed tomography; LDH = lactate dehydrogenase; PET = positron emission tomography.
*—Follow-up for the first five years is specifically for the diagnosis of melanoma. Annual follow-up for life is recommended to be performed by a dermatologist for routine skin cancer screening.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms cutaneous melanoma, incidence, diagnosis, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Cochrane Database of Systematic Reviews; U.S. Preventive Services Task Force; UpToDate; Surveillance, Epidemiology, and End Results (SEER) Program; National Center for Health Statistics; and the National Cancer Data Base. Search date: July 15, 2010.
