
Am Fam Physician. 2012;85(2):195-196
Related: Putting Prevention into Practice
Summary of Recommendation and Evidence
The U.S. Preventive Services Task Force (USPSTF) recommends prophylactic ocular topical medication for all newborns for the prevention of gonococcal ophthalmia neonatorum (Table 1). A recommendation.
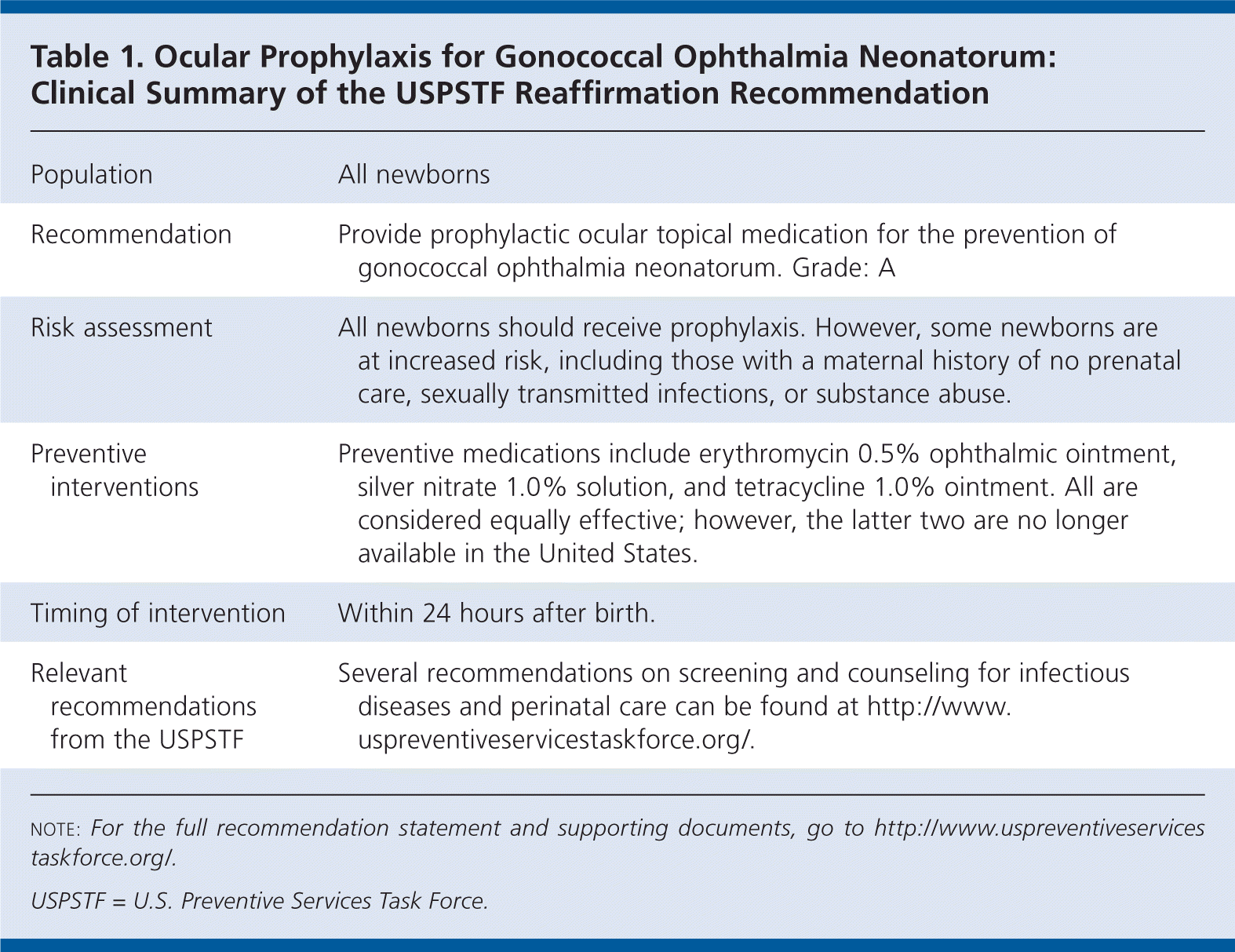
| Population | All newborns |
| Recommendation | Provide prophylactic ocular topical medication for the prevention of gonococcal ophthalmia neonatorum. Grade: A |
| Risk assessment | All newborns should receive prophylaxis. However, some newborns are at increased risk, including those with a maternal history of no prenatal care, sexually transmitted infections, or substance abuse. |
| Preventive interventions | Preventive medications include erythromycin 0.5% ophthalmic ointment, silver nitrate 1.0% solution, and tetracycline 1.0% ointment. All are considered equally effective; however, the latter two are no longer available in the United States. |
| Timing of intervention | Within 24 hours after birth. |
| Relevant recommendations from the USPSTF | Several recommendations on screening and counseling for infectious diseases and perinatal care can be found at http://www.uspreventiveservicestaskforce.org/. |
Rationale
Importance. Gonococcal ophthalmia neonatorum develops in approximately 28 percent of newborns delivered to women with gonorrheal disease in the United States. Identifying and treating the infection are important because gonococcal ophthalmia neonatorum can result in corneal scarring, ocular perforation, and blindness.
Recognition of risk status. The USPSTF recommends that all newborns receive prophylaxis; however, some newborns are at increased risk of gonococcal ophthalmia neonatorum. Newborns at increased risk include those with a maternal history of sexually transmitted infections, substance abuse, or no prenatal care.
Benefits of risk assessment and preventive medication. There is convincing evidence that blindness due to gonococcal ophthalmia neonatorum has become rare in the United States since the implementation of universal prophylaxis of newborns.
Harms of risk assessment and preventive medication. There is convincing evidence that universal prophylaxis of newborns is not associated with serious harms.
USPSTF assessment. The USPSTF concludes that there is high certainty that the net benefit is substantial for topical ocular prophylaxis for all newborns for the prevention of gonococcal ophthalmia neonatorum.
Clinical Considerations
PATIENT POPULATION
This clinical recommendation applies to all newborns.
PREVENTIVE MEDICATION
Prophylactic regimens using tetracycline 1.0% or erythromycin 0.5% ophthalmic ointment are equally effective in the prevention of gonococcal ophthalmia neonatorum; however, the only drug approved by the U.S. Food and Drug Administration for this indication is erythromycin 0.5% ophthalmic ointment. Tetracycline ophthalmic ointment and silver nitrate are no longer available in the United States. A 2.5% solution of povidone-iodine may be useful in preventing ophthalmia neonatorum, but it has not been approved for use in the United States.
OPTIMAL TIMING
Prophylaxis should be provided within 24 hours after birth.
