
Am Fam Physician. 2012;85(4):352-358
A more recent article on acute respiratory distress syndrome is available.
Patient information: See related handout on acute respiratory distress syndrome, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Acute respiratory distress syndrome manifests as rapidly progressive dyspnea, tachypnea, and hypoxemia. Diagnostic criteria include acute onset, profound hypoxemia, bilateral pulmonary infiltrates, and the absence of left atrial hypertension. Acute respiratory distress syndrome is believed to occur when a pulmonary or extrapulmonary insult causes the release of inflammatory mediators, promoting neutrophil accumulation in the microcirculation of the lung. Neutrophils damage the vascular endothelium and alveolar epithelium, leading to pulmonary edema, hyaline membrane formation, decreased lung compliance, and difficult air exchange. Most cases of acute respiratory distress syndrome are associated with pneumonia or sepsis. It is estimated that 7.1 percent of all patients admitted to an intensive care unit and 16.1 percent of all patients on mechanical ventilation develop acute lung injury or acute respiratory distress syndrome. In-hospital mortality related to these conditions is between 34 and 55 percent, and most deaths are due to multiorgan failure. Acute respiratory distress syndrome often has to be differentiated from congestive heart failure, which usually has signs of fluid overload, and from pneumonia. Treatment of acute respiratory distress syndrome is supportive and includes mechanical ventilation, prophylaxis for stress ulcers and venous thromboembolism, nutritional support, and treatment of the underlying injury. Low tidal volume, high positive end-expiratory pressure, and conservative fluid therapy may improve outcomes. A spontaneous breathing trial is indicated as the patient improves and the underlying illness resolves. Patients who survive acute respiratory distress syndrome are at risk of diminished functional capacity, mental illness, and decreased quality of life; ongoing care by a primary care physician is beneficial for these patients.
Acute respiratory distress syndrome (ARDS) is a rapidly progressive disorder that initially manifests as dyspnea, tachypnea, and hypoxemia, then quickly evolves into respiratory failure. The American-European Consensus Conference (AECC) has published diagnostic criteria for ARDS: acute onset; ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) of 200 or less, regardless of positive end-expiratory pressure; bilateral infiltrates seen on frontal chest radiograph; and pulmonary artery wedge pressure of 18 mm Hg or less when measured, or no clinical evidence of left atrial hypertension.1 Acute lung injury is a slightly less severe syndrome characterized by less profound hypoxemia but otherwise similar diagnostic criteria to ARDS.1 Because more than one-half of intensive care units (ICUs) in the United States are not staffed with intensivists,2 many primary care physicians provide care for patients with ARDS or acute lung injury.
| Clinical recommendation | Evidence rating | References |
| When mechanical ventilation is required, patients with ARDS should be started at lower tidal volumes (6 mL per kg) instead of at traditional volumes (10 to 15 mL per kg). | A | 21, 22 |
| Higher positive end-expiratory pressure values (12 to 18 or more cm H2O) should be considered for initial mechanical ventilation in patients with ARDS. | B | 23 |
| Conservative fluid therapy (targeting lower central pressures) in patients with ARDS may be associated with decreased days on a ventilator and increased days outside the intensive care unit. | B | 24 |
| Pulmonary artery catheters should not be used for the routine management of ARDS. | A | 25, 26 |
| Surfactant therapy does not improve mortality in adults with ARDS. | A | 27 |
ARDS = acute respiratory distress syndrome.
A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series. For information about the SORT evidence rating system, go to https://www.aafp.org/afpsort.xml.
Pathophysiology
The pathophysiology of ARDS is not completely understood. Initially, a direct pulmonary or indirect extrapulmonary insult is believed to cause a proliferation of inflammatory mediators that promote neutrophil accumulation in the microcirculation of the lung. These neutrophils activate and migrate in large numbers across the vascular endothelial and alveolar epithelial surfaces, releasing proteases, cytokines, and reactive oxygen species. This migration and mediator release lead to pathologic vascular permeability, gaps in the alveolar epithelial barrier, and necrosis of type I and II alveolar cells. This, in turn, leads to the pulmonary edema, hyaline membrane formation, and loss of surfactant that decrease pulmonary compliance and make air exchange difficult. Subsequent infiltration of fibroblasts can lead to collagen deposition, fibrosis, and worsening disease. Figure 1 is a radiograph of a patient with ARDS showing the bilateral air space opacification that results from these processes.
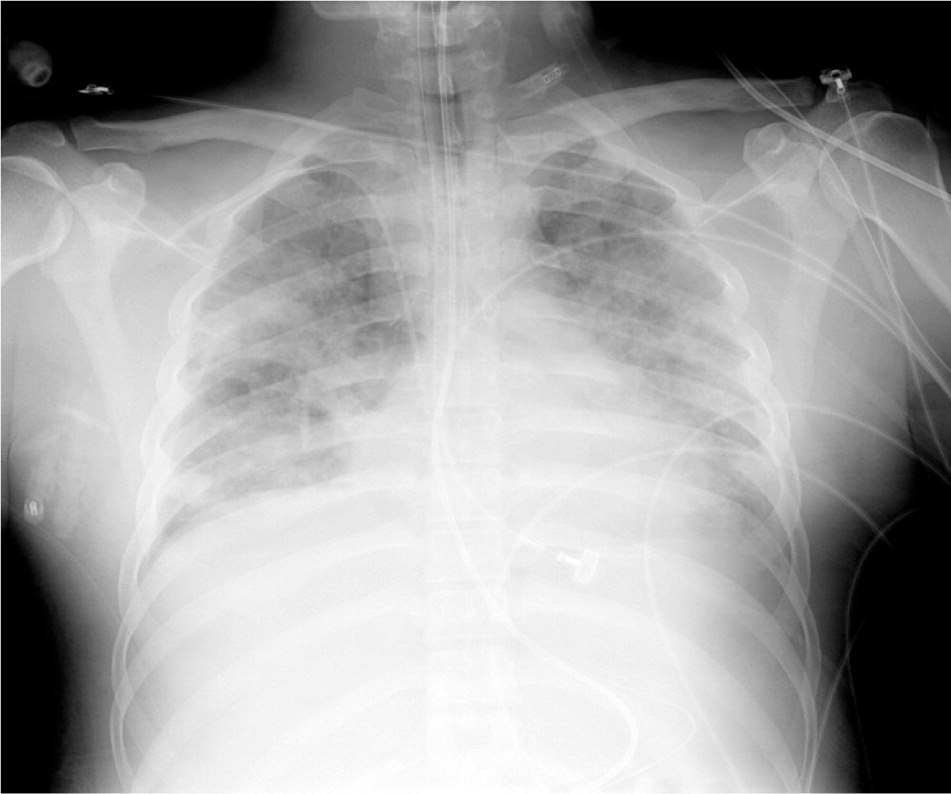
In recovery, multiple actions occur simultaneously. Anti-inflammatory cytokines deactivate inciting neutrophils, which then undergo apoptosis and phagocytosis. Type II alveolar cells proliferate and differentiate into type I cells, reestablishing the integrity of the epithelial lining and creating an osmotic gradient that draws fluid out of the alveoli and into the pulmonary microcirculation and lung lymphatics. Simultaneously, alveolar cells and macrophages remove protein compounds from the alveoli, allowing the lungs to recover.3,4
Risk Factors and Incidence
Most cases of ARDS in adults are associated with pulmonary sepsis (46 percent) or nonpulmonary sepsis (33 percent).5,6 Risk factors include those causing direct lung injury (e.g., pneumonia, inhalation injury, pulmonary contusion) and those causing indirect lung injury (e.g., nonpulmonary sepsis, burns, transfusion-related acute lung injury).1,7 Risk factors in children are similar to those in adults, with the addition of age-specific disorders, such as respiratory syncytial virus infection and near drowning aspiration injury.8 Table 1 includes signs and symptoms suggesting specific causes of ARDS.9,10
| Signs/symptoms | Possible cause |
|---|---|
| Most common | |
| Productive cough, fever, pleuritic chest pain | Pneumonia |
| Infection plus some of the following findings: temperature > 100.9°F (38.3°C) or < 96.8°F (36°C); pulse > 90 beats per minute; tachypnea; altered mental status; white blood cell count > 12,000 per mm3 (12 × 109 per L), < 4,000 mm3 (4 × 109 per L), or > 10 percent immature forms; elevated C-reactive protein level; arterial hypotension; acute oliguria; hyperlactatemia | Sepsis |
| Less common | |
| History of institutionalization or mental retardation, decreased Glasgow Coma Scale score | Aspiration |
| History of drug abuse, especially inhalational | Drug toxicity |
| Facial burns, singed eyebrows, carbonaceous sputum, history of working near organic solvents or toxic chemicals | Inhalation injury |
| History of needing water rescue, hypothermia | Near drowning |
| Fever, rhinorrhea, cough, history of prematurity or congenital heart disease | Respiratory syncytial virus |
| Symptoms of acute lung injury and blood transfusion within the previous six hours | Transfusion-related acute lung injury |
| Bruising over the chest wall, associated injuries, history of motor vehicle crash or fall from a height | Trauma |
Recent studies indicate that the incidence of adult acute lung injury and ARDS is 22 to 86 cases per 100,000 person-years and up to 64 cases per 100,000 person-years, respectively.5,11 A large, prospective European trial estimated that 7.1 percent of patients admitted to an ICU and 16.1 percent of all patients on mechanical ventilation develop acute lung injury or ARDS.12 The in-hospital mortality rate for these conditions is estimated to be between 34 and 55 percent.5,12,13 Risk factors for mortality include increasing age, worsening multiorgan dysfunction, presence of pulmonary and nonpulmonary comorbidities, higher Acute Physiology and Chronic Health Evaluation (APACHE) II score, and acidosis. Most ARDS-related deaths are due to multiorgan failure. Refractory hypoxemia accounts for only 16 percent of ARDS-related deaths.14
In children, ARDS is less common and less likely to lead to death. In a 2009 study of patients six months to 15 years of age, the reported incidences of acute lung injury and ARDS were 9.5 and 12.8 per 100,000 person-years, respectively, with a combined in-hospital mortality rate of 18 percent.8
Differential Diagnosis
Because the presenting symptoms of ARDS are nonspecific, physicians must consider other respiratory, cardiac, infectious, and toxic etiologies (Table 215–17 ). Patient history (e.g., comorbidities, exposures, medications) in conjunction with a physical examination focusing on the respiratory and cardiovascular systems can help narrow the differential diagnosis and determine the optimal course of treatment.

| Condition | Clues to diagnosis |
|---|---|
| More common | |
| Asthma | Cough, wheeze, response to bronchodilator |
| Chronic obstructive pulmonary disease | Decreased air movement, prolonged expiratory phase |
| Congestive heart failure | Jugular venous distension, peripheral edema, third heart sound |
| Pneumonia* | Productive cough, fever, pleuritic chest pain |
| Less common | |
| Acute eosinophilic pneumonia | Fever, cough, diffuse infiltrates, increased eosinophils on bronchoalveolar lavage |
| Hypersensitivity pneumonitis | Acute onset; exposure to inciting organic antigen, such as those found in bird feathers, molds, and dust |
| Pneumothorax | Acute onset of dyspnea, pleuritic chest pain; tall and thin body habitus |
| Salicylate toxicity | History of suicide attempt, hyperventilation, tachycardia, seizure |
| Sepsis* | Fever, tachypnea, tachycardia, elevated or depressed white blood cell count |
Often, ARDS must be differentiated from congestive heart failure and pneumonia (Table 318,19 ). Congestive heart failure is characterized by fluid overload, whereas patients diagnosed with ARDS, by definition, do not show signs of left atrial hypertension or overt volume overload. Patients with congestive heart failure may have edema, jugular venous distension, third heart sound, an elevated brain natriuretic peptide level, and a salutary response to diuretics. Patients with ARDS would not be expected to have these findings.
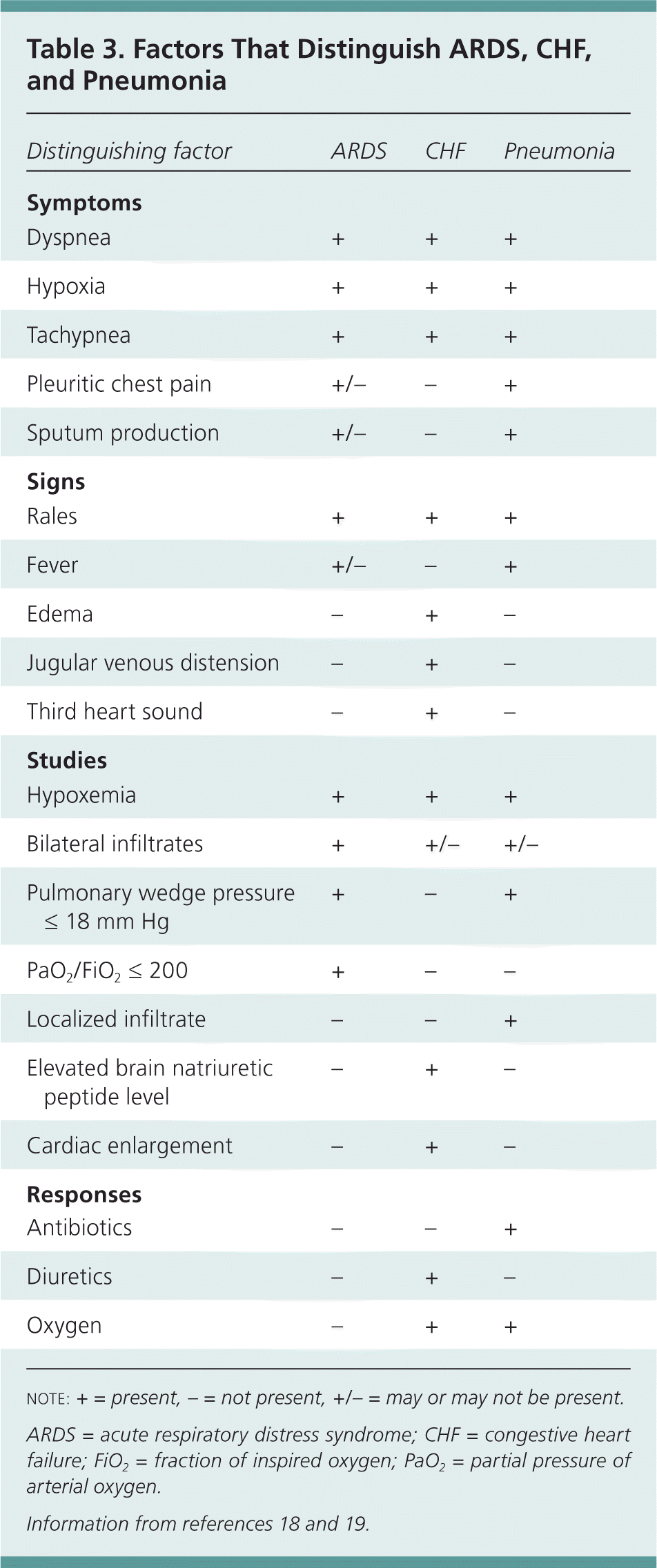
| Distinguishing factor | ARDS | CHF | Pneumonia |
|---|---|---|---|
| Symptoms | |||
| Dyspnea | + | + | + |
| Hypoxia | + | + | + |
| Tachypnea | + | + | + |
| Pleuritic chest pain | +/– | – | + |
| Sputum production | +/– | – | + |
| Signs | |||
| Rales | + | + | + |
| Fever | +/– | – | + |
| Edema | – | + | – |
| Jugular venous distension | – | + | – |
| Third heart sound | – | + | – |
| Studies | |||
| Hypoxemia | + | + | + |
| Bilateral infiltrates | + | +/– | +/– |
| Pulmonary wedge pressure ≤ 18 mm Hg | + | – | + |
| PaO2/FiO2 ≤ 200 | + | – | – |
| Localized infiltrate | – | – | + |
| Elevated brain natriuretic peptide level | – | + | – |
| Cardiac enlargement | – | + | – |
| Responses | |||
| Antibiotics | – | – | + |
| Diuretics | – | + | – |
| Oxygen | – | + | + |
Because pneumonia is a leading cause of ARDS, distinguishing patients with uncomplicated pneumonia from those who have pneumonia complicated by ARDS presents a greater diagnostic challenge. In general, a patient with uncomplicated pneumonia may have signs of systemic and pulmonary inflammation (i.e., fever, chills, fatigue, sputum production, pleuritic chest pain, and localized or multifocal infiltrates); accompanying hypoxia should respond to oxygen administration. If hypoxia does not correct with oxygen administration, ARDS should be suspected and confirmed based on AECC diagnostic criteria. In those with combined pneumonia and ARDS, treatment entails antibiotics and ventilator management.
Treatment and Support
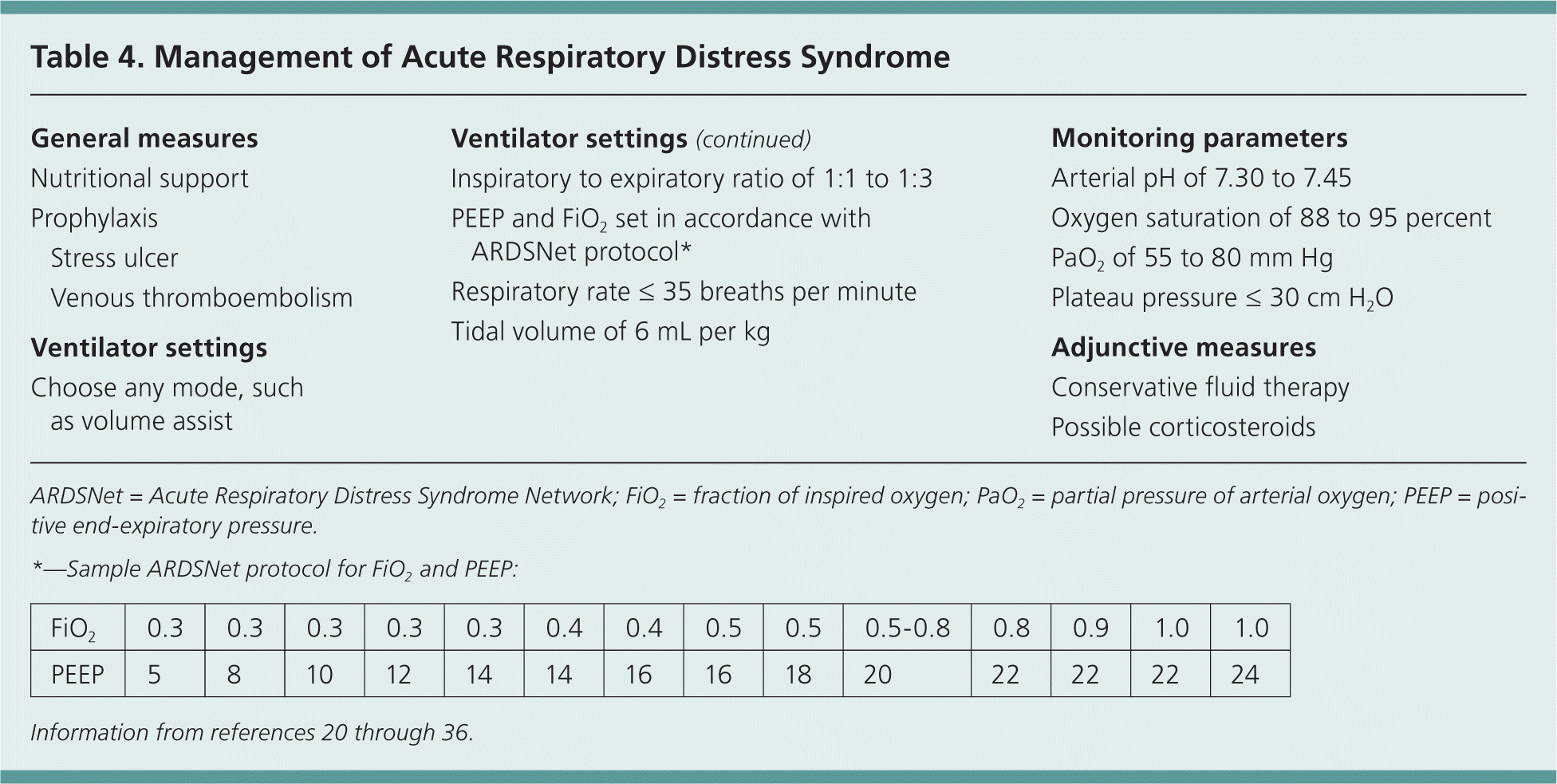
| General measures | |
| Nutritional support | |
| Prophylaxis | |
| Stress ulcer | |
| Venous thromboembolism | |
| Ventilator settings | |
| Choose any mode, such as volume assist | |
| Inspiratory to expiratory ratio of 1:1 to 1:3 | |
| PEEP and FiO2 set in accordance with ARDSNet protocol* | |
| Respiratory rate ≤ 35 breaths per minute | |
| Tidal volume of 6 mL per kg | |
| Monitoring parameters | |
| Arterial pH of 7.30 to 7.45 | |
| Oxygen saturation of 88 to 95 percent | |
| PaO2 of 55 to 80 mm Hg | |
| Plateau pressure ≤ 30 cm H2O | |
| Adjunctive measures | |
| Conservative fluid therapy | |
| Possible corticosteroids | |
MECHANICAL VENTILATION
Most patients with ARDS need sedation, intubation, and ventilation while the underlying injury is treated. Any ventilator mode may be used, according to the Surviving Sepsis Clinical Practice Guideline and the National Heart, Lung, and Blood Institute's ARDS Network (ARDSNet).20,21 Respiratory rate, expiratory time, positive end-expiratory pressure, and FiO2 are set in accordance with ARDSNet protocols. Settings are adjusted to maintain an oxygen saturation of 88 to 95 percent and a plateau pressure of 30 cm H2O or less to avoid barotrauma. Clinical practice guidelines recommend maintaining an arterial pH of 7.30 to 7.45, although patients in some research trials have tolerated permissive hypercapnia and a pH as low as 7.15.20–22
Evidence has shown that starting with low tidal volumes of 6 mL per kg is superior to starting with traditional tidal volumes of 10 to 15 mL per kg (number needed to treat = 11.4).21,22 Similarly, higher positive end-expiratory pressure values (12 cm H2O or more) are associated with decreased mortality compared with lower values of 5 to 12 cm H2O (number needed to treat = 20).23 Conservative fluid therapy (titrated to lower central pressures) has been associated with decreased days on a ventilator and increased days outside the ICU.24 Because of the potential complications of pulmonary artery and central venous catheters, they are not used routinely and should be administered only by those with training and experience.25,26
PHARMACOLOGIC THERAPIES
Pharmacologic options for the treatment of ARDS are limited. Although surfactant therapy may be helpful in children with ARDS, a Cochrane review did not find it to be beneficial in adults.27 The use of corticosteroids is controversial. Randomized controlled trials and cohort studies tend to support early use of corticosteroids (with dosages of methylprednisolone [Solu-Medrol] ranging from 1 to 120 mg per kg per day) for decreasing the number of days on a ventilator; however, no consistent mortality benefit has been shown with this therapy.28,29 A medical intensivist should be consulted when considering the use of corticosteroids.
In addition to ventilatory measures, patients with ARDS should receive low-molecular-weight heparin (40 mg of enoxaparin [Lovenox] or 5,000 units of dalteparin [Fragmin] subcutaneously per day) or low-dose, unfractionated heparin (5,000 units subcutaneously twice daily) to prevent venous thromboembolism, unless contraindicated.30,31 Patients should also be on stress ulcer prophylaxis with an agent such as sucralfate (Carafate; 1 g orally or via nasogastric tube four times daily), ranitidine (Zantac; 150 mg orally or via nasogastric tube twice daily, 50 mg intravenously every six to eight hours, or a 6.25-mg-per-hour continuous intravenous infusion), or omeprazole (Prilosec; 40 mg orally, intravenously, or via nasogastric tube daily).32–35 Finally, patients should receive nutritional support, preferably enteral, within 24 to 48 hours of admission to the ICU.36
VENTILATOR WEANING
As the underlying illness resolves and the patient improves, a spontaneous breathing trial is indicated. To be eligible for a trial, the patient should be hemodynamically stable and able to meet oxygen requirements through noninvasive methods. Spontaneous breathing trials are conducted over one to two hours. Extubation is more likely to be successful if the patient remains hemodynamically stable with good ventilatory parameters.20 Standardized weaning protocols have been used to reduce the duration of mechanical ventilation.38 Table 5 summarizes the eligibility criteria for starting a spontaneous breathing trial and parameters for weaning the patient from the ventilator.20,38
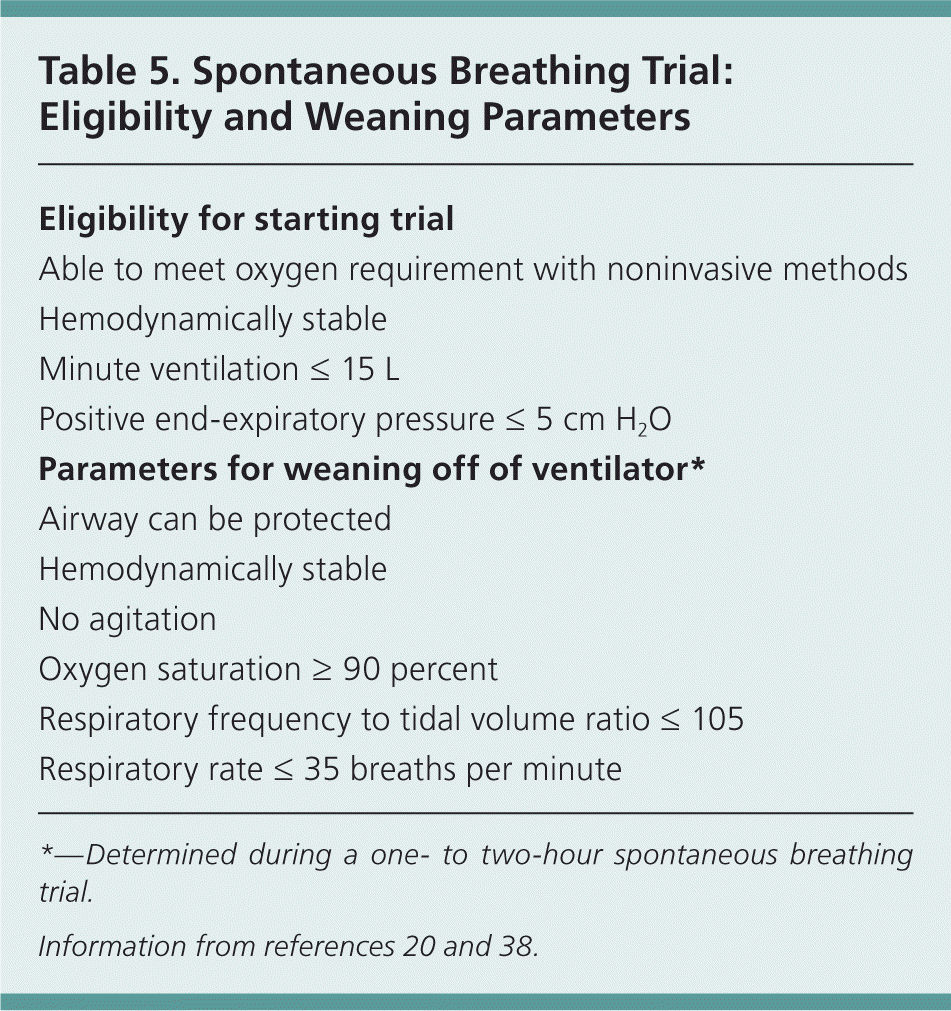
| Eligibility for starting trial |
| Able to meet oxygen requirement with noninvasive methods |
| Hemodynamically stable |
| Minute ventilation ≤ 15 L |
| Positive end-expiratory pressure ≤ 5 cm H2O |
| Parameters for weaning off of ventilator* |
| Airway can be protected |
| Hemodynamically stable |
| No agitation |
| Oxygen saturation ≥ 90 percent |
| Respiratory frequency to tidal volume ratio ≤ 105 |
| Respiratory rate ≤ 35 breaths per minute |
MOBILIZATION THERAPY
Post-ARDS Primary Care
The care of patients with ARDS does not end after the acute illness and often prolonged hospitalization. After discharge from the ICU, patients with ARDS tend to have a lower quality of life than they did before,41 significant weakness from neuropathy or myopathy,42 persistent cognitive impairment,43 and delayed return to work.44 Mortality at three years is higher in those who required mechanical ventilation in the ICU (57.3 percent) compared with those who did not require ventilation in the ICU (38.3 percent) and those who were not admitted to the ICU (14.9 percent).45
Patients with ARDS or who required prolonged ventilation (greater than seven days) in the ICU tend to have a lower quality of life41 and more weakness42 at discharge than those without ARDS or the need for prolonged ventilation. Psychiatric illness is also widely prevalent after ARDS, with 17 to 43 percent of survivors reporting depression, 21 to 35 percent reporting posttraumatic stress disorder, and 23 to 48 percent reporting anxiety.46 Risk factors for poor outcomes include a higher APACHE II score, acquisition of illness in the ICU, longer time to resolution of lung and multiorgan dysfunction, and use of systemic corticosteroids.47
Not all of the deleterious health effects of hospitalization for ARDS resolve over time. Although lung function approaches normal at five years, six-minute walking distance, physical function, and quality-of-life measures often are still decreased. In addition, many patients report social isolation and sexual dysfunction, and more than one-half of patients report persistent depression, anxiety, or both.48
Because the burden of illness is significant in the more than 100,000 persons who survive ARDS each year,5 it is imperative that primary care physicians initiate, coordinate, and monitor continuing services for these patients. Physicians should assess functional status at hospital follow-up and subsequent visits, ensuring that the resources of the multidimensional health care team (e.g., physical and occupational therapy, rehabilitation nursing, home health care, subspecialty colleagues) are used to promote optimal health and function. In addition, primary care physicians should screen for mental health disturbances and treat or refer as needed.
Data Sources: A PubMed search was completed using the key words acute respiratory distress syndrome and acute lung injury. The search included meta-analyses, randomized controlled trials, clinical trials, epidemiologic studies, and reviews. Also searched were the Cochrane Database of Systematic Reviews, the National Guidelines Clearinghouse, and Essential Evidence Plus. Search date: spring 2010 to summer 2011.
