
A more recent article on smoking cessation is available.
Am Fam Physician. 2012;85(6):591-598
Patient information: See related handout on smoking cessation, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Cigarette smoking causes significant morbidity and mortality in the United States. Physicians can use the five A's framework (ask, advise, assess, assist, arrange) to promote smoking cessation. All patients should be asked about tobacco use and assessed for motivation to quit at every clinical encounter. Physicians should strongly advise patients to quit smoking, and use motivational interviewing techniques for patients who are not yet willing to stop smoking. Clinical contacts with unmotivated patients should emphasize the rewards and relevance of quitting, as well as the risks of smoking and anticipated barriers to abstinence. These messages should be repeated at every opportunity. Appropriate patients should be offered pharmacologic assistance in quitting, such as nicotine replacement therapies, bupropion, and varenicline. Use of pharmacologic support during smoking cessation can double the rate of successful abstinence. Using more than one type of nicotine replacement therapy (“patch plus” method) and combining these therapies with bupropion provide additional benefit. However, special populations pose unique challenges in pharmacotherapy for smoking cessation. Nicotine replacement therapies increase the risk of birth defects and should not be used during pregnancy. They are usually safe in patients with cardiovascular conditions, except for those with unstable angina or within two weeks of a coronary event. Varenicline may increase the risk of coronary events. Nicotine replacement therapies are safe for use in adolescents; however, they are less effective than in adults. Physicians also should arrange to have repeated contact with smokers around their quit date to reinforce cessation messages.
Cigarette smoking is a major modifiable health risk factor in the United States, significantly contributing to deaths from cancer and cardiovascular and pulmonary diseases. Although it is estimated that smoking-related illnesses lead to 443,000 premature deaths and almost $100 billion in lost productivity each year,1 one in five American adults still smokes regularly (22 percent of men, 17.5 percent of women).2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| All adults should be screened routinely for tobacco use. | A | 6 |
| All smokers should be encouraged to quit at every clinical contact. | A | 4, 20 |
| Motivational interventions should be used with patients who are not yet ready to quit smoking. | A | 4, 25 |
| Physicians should encourage appropriate patients to use effective medications for treatment of tobacco dependence to improve quit rates. | A | 4, 27–29 |
| Heavy smokers should be encouraged to use higher dosages of a nicotine replacement therapy, or more than one form (“patch plus” regimen). | B | 4 |
| Pregnant smokers should be offered person-to-person psychosocial interventions that exceed minimal advice to quit. | B | 4 |
| Sustained-release bupropion (Zyban) or a nicotine replacement therapy (particularly gum and lozenges) may be more appropriate for smokers who are concerned about weight gain after quitting. | C | 4 |
Five A's Counseling Strategy
Physicians should address smoking cessation with all patients who use tobacco.6 Although intensive group and individual psychological counseling are effective in helping smokers achieve abstinence, most smokers are not interested in participating in these types of interventions.7,8 The five A's framework (ask, advise, assess, assist, arrange) has been developed to allow physicians to incorporate smoking cessation counseling into busy clinical practices.4 Table 1 describes the five A's framework for smoking cessation counseling.4,9–19
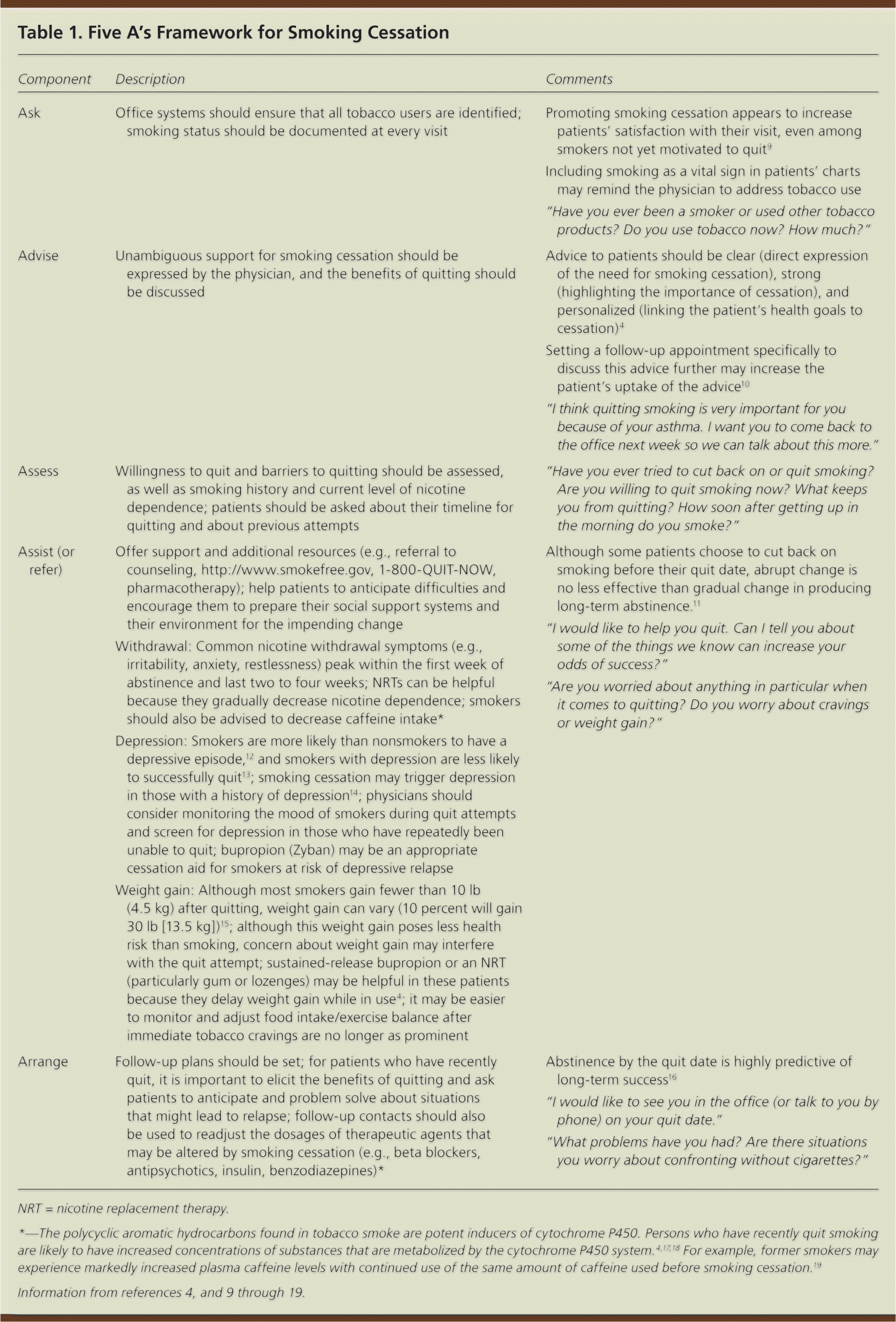
| Component | Description | Comments |
|---|---|---|
| Ask |
|
|
| Advise |
|
|
| Assess |
|
|
| Assist (or refer) |
|
|
| Arrange |
|
|
ASK
Adding smoking status as a vital sign to all patients' charts increases the likelihood that physicians will address tobacco use as a risk behavior with smokers and provide them with cessation-related advice.20
ADVISE
Even brief physician advice may prompt an additional 1 to 3 percent of patients to attempt cessation and improve quit rates compared with patients who receive no advice (relative risk = 1.7).21
ASSESS
Behavior change can be conceptualized into five progressive stages: precontemplation, contemplation, preparation, action, and maintenance (Table 2).23 Although tailoring interventions to a patient's stage of change may not be necessary,24 these stages emphasize that not all patients are equally motivated to quit smoking, motivation is malleable, and patients can be assisted toward behavior change through physician intervention.
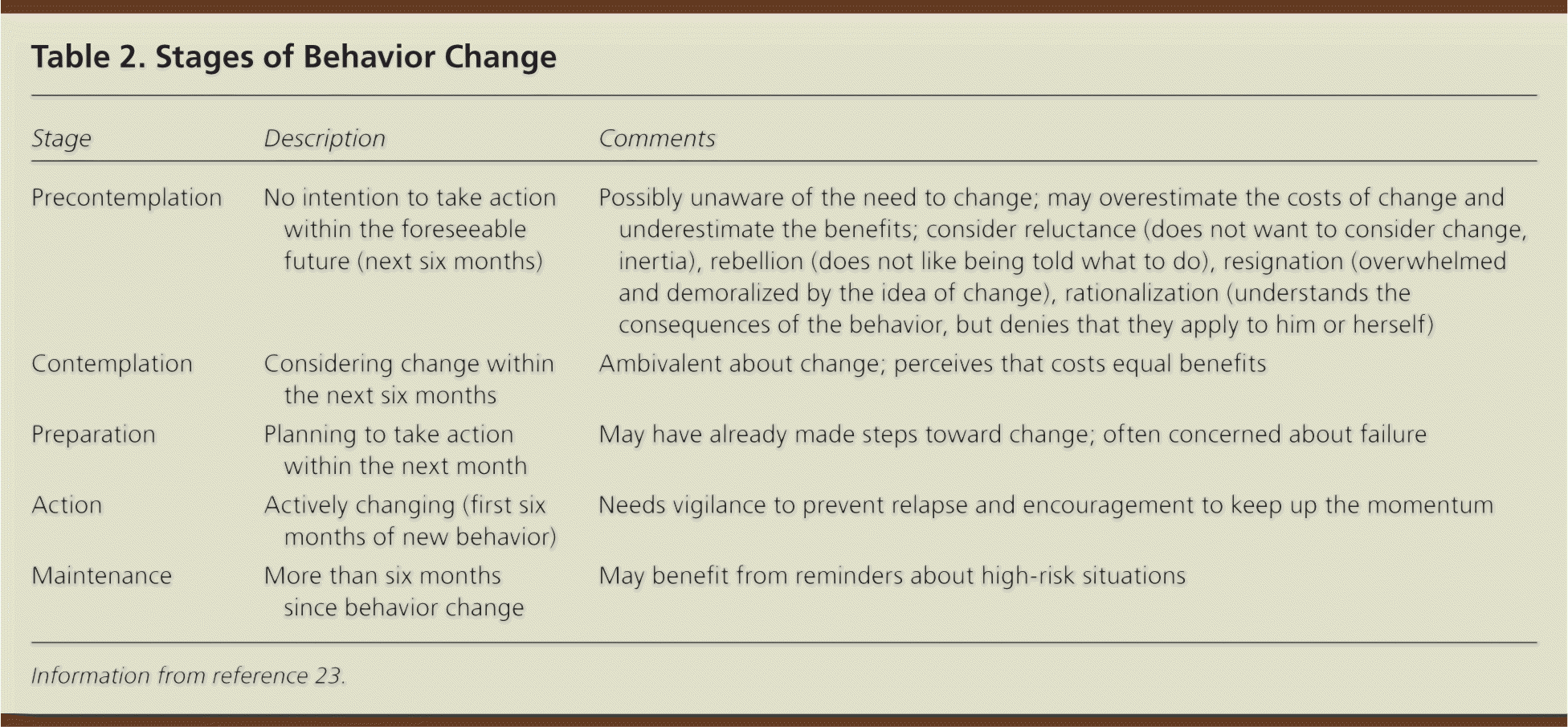
| Stage | Description | Comments |
|---|---|---|
| Precontemplation | No intention to take action within the foreseeable future (next six months) | Possibly unaware of the need to change; may overestimate the costs of change and underestimate the benefits; consider reluctance (does not want to consider change, inertia), rebellion (does not like being told what to do), resignation (overwhelmed and demoralized by the idea of change), rationalization (understands the consequences of the behavior, but denies that they apply to him or herself) |
| Contemplation | Considering change within the next six months | Ambivalent about change; perceives that costs equal benefits |
| Preparation | Planning to take action within the next month | May have already made steps toward change; often concerned about failure |
| Action | Actively changing (first six months of new behavior) | Needs vigilance to prevent relapse and encouragement to keep up the momentum |
| Maintenance | More than six months since behavior change | May benefit from reminders about high-risk situations |
Confrontational interactions with patients ambivalent about behavior change are ineffective. Motivational interventions, by contrast, explore a patient's ambivalence to smoking cessation in an empathetic, questioning manner, which respects the patient's autonomy and builds self-efficacy.22 Motivational interventions, especially those in which physicians take a central role in the counseling, are more effective than brief advice and usual care in promoting smoking cessation.25 The Agency for Healthcare Research and Quality has identified several components of discussion to enhance patients' motivation to stop smoking. These components are the five R's (relevance, risks, rewards, roadblocks, repeat; Table 3).4,26

| Component | Description | Examples |
|---|---|---|
| Relevance | Encourage the patient to identify reasons to stop smoking that are personally relevant | Pregnancy, personal or family risk of disease, person in the household with asthma |
| Risks | Advise the patient of the harmful effects of continued smoking, both to the patient and to others, incorporating aspects of the personal and family history whenever possible | Effects on the patient and the patient's family, friends, and coworkers; measuring “lung age”* through spirometry can help personalize risk26 |
| Rewards | Ask the patient to identify the benefits of smoking cessation | Improved health, financial savings from not buying cigarettes, decreased cigarette odor |
| Roadblocks | Explore the barriers to cessation that the patient may encounter | Presence of other smokers in the home or workplace, history of failed quit attempts or severe withdrawal symptoms, stress, psychiatric comorbidity, low motivation, weight gain, enjoyment of smoking |
| Repeat | Include aspects of the five R's in each clinical contact with unmotivated smokers | — |
ASSIST (OR REFER)
Asking patients who are willing to quit to set a quit date can prompt change, and physicians should help patients anticipate obstacles to cessation. Nicotine withdrawal symptoms, depression, and weight gain are specific areas in which patients may benefit from clinical guidance.4
ARRANGE FOR FOLLOW-UP
Patients should be contacted around the time of their quit date to be congratulated on their (presumed) abstinence. Contacting patients at least four more times to support their smoking-cessation attempts increases abstinence rates.4
Medications
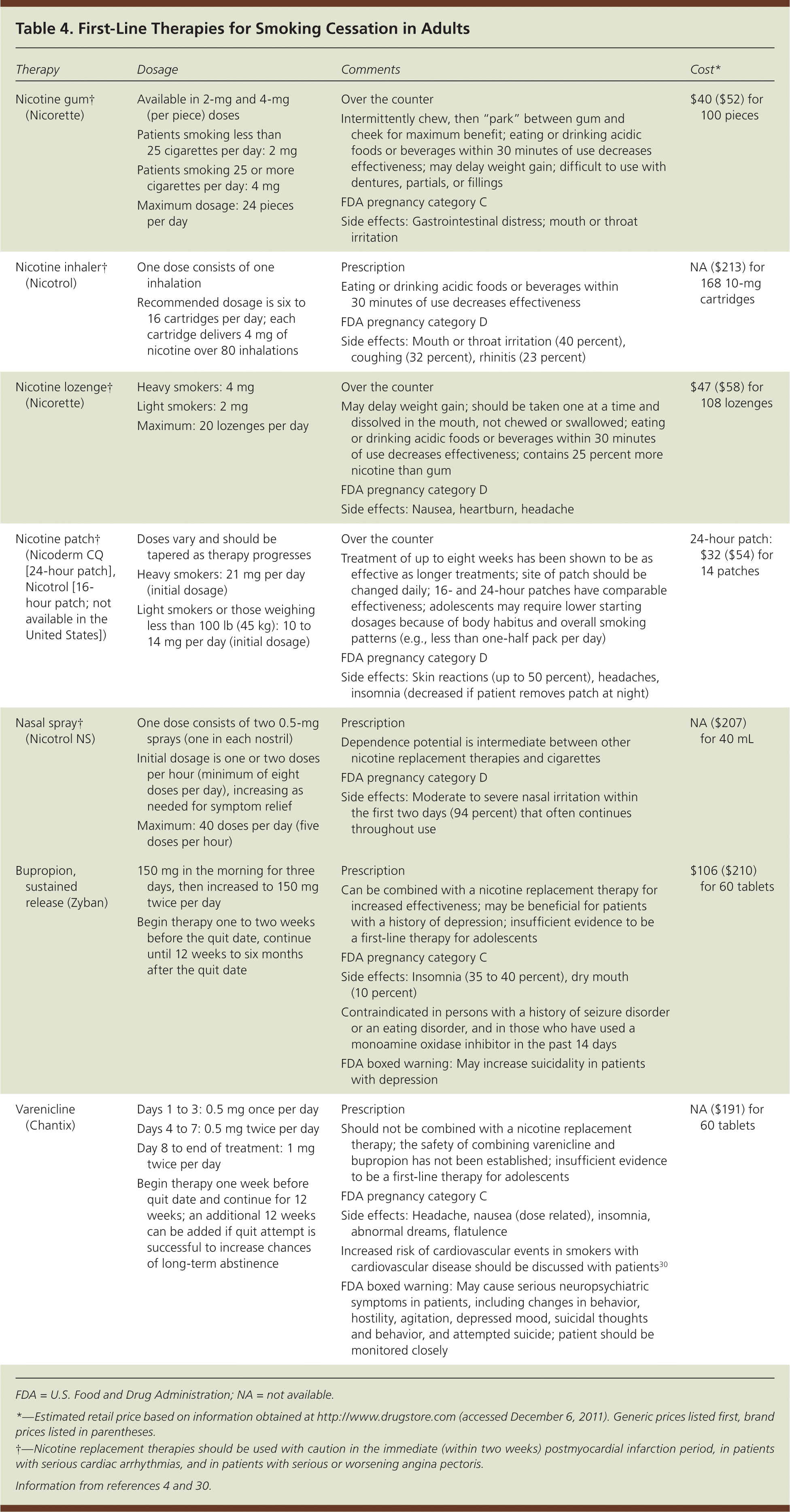
| Therapy | Dosage | Comments | Cost* | ||
|---|---|---|---|---|---|
| Nicotine gum† (Nicorette) |
|
| $40 ($52) for 100 pieces | ||
| Nicotine inhaler† (Nicotrol) |
|
| NA ($213) for 168 10-mg cartridges | ||
| Nicotine lozenge† (Nicorette) |
|
| $47 ($58) for 108 lozenges | ||
| Nicotine patch† (Nicoderm CQ [24-hour patch], Nicotrol [16-hour patch; not available in the United States]) |
|
| 24-hour patch: $32 ($54) for 14 patches | ||
| Nasal spray† (Nicotrol NS) |
|
| NA ($207) for 40 mL | ||
| Bupropion, sustained release (Zyban) |
|
| $106 ($210) for 60 tablets | ||
| Varenicline (Chantix) |
|
| NA ($191) for 60 tablets | ||
NICOTINE REPLACEMENT THERAPIES
The goal of nicotine replacement therapies (NRTs) is to relieve cravings for nicotine and reduce nicotine withdrawal symptoms. NRTs are available as slow-release skin patches and in more rapidly acting forms (i.e., chewing gum, nasal spray, inhalers, and lozenges), which deliver nicotine to the brain more quickly than skin patches but more slowly than smoking cigarettes. A Cochrane review of 132 trials concluded that all forms of NRTs increase the chances of quitting successfully by 50 to 70 percent.27
Heavy smokers should be encouraged to use higher dosages of an NRT or try a “patch plus” method, using the nicotine patch to provide a base level of slowly delivered nicotine and adding a more rapidly acting NRT to control breakthrough cravings. This regimen is safe because smokers typically obtain less nicotine than through smoking, and it is more effective than using a single NRT.4
NICOTINIC RECEPTOR AGONIST
Varenicline (Chantix) is a selective alpha4-beta2 nicotinic receptor partial agonist that reduces cravings and withdrawal symptoms while blocking the binding of smoked nicotine.28 Varenicline increases the chances of a successful quit attempt two- to threefold compared with no pharmacologic assistance.31 In a direct comparison, varenicline was superior to bupropion in promoting abstinence.32 However, new data suggest increased risk of coronary events with varenicline.30
BUPROPION
Initially developed as an antidepressant, bupropion has been shown to be effective as a smoking cessation aid. A Cochrane review of 19 randomized trials showed that bupropion doubles the odds of smoking cessation compared with placebo.29
SECOND-LINE THERAPIES

| Therapy | Comments |
|---|---|
| Acupuncture | Acupuncture, acupressure, laser acupuncture, and electroacupuncture all have been proposed as cures for nicotine addiction or as a means to reduce withdrawal symptoms |
| A Cochrane review did not find consistent evidence that these therapies are more beneficial for smoking cessation than no treatment or sham acupuncture33 | |
| Exercise | Small, heterogeneous studies provide little evidence that exercise improves quit rates34; however, it may be useful to promote weight control after smoking cessation35 |
| Hypnotherapy | A Cochrane review found a lack of evidence that hypnotherapy is beneficial compared with other therapies or no treatment36 |
| Internet-based interventions (e.g., http://www.smokefree.gov) | A Cochrane review of 20 randomized and quasi-randomized studies did not find conclusive evidence of benefit, especially at long-term follow-up37; Web sites providing personally tailored information and repeated automated contacts may be beneficial as an adjunct to other cessation strategies4 |
| Telephone quitlines (e.g., 800-QUIT-NOW) | Physician encouragement to use quitlines has a two to three times greater effect on smoking cessation than counseling alone in the primary care setting38; the use of quitlines results in a relative risk of cessation of 1.3739 |
Special Populations
PREGNANT WOMEN
Tobacco is a known carcinogen that can harm a developing fetus. Women who quit smoking before pregnancy or in early pregnancy significantly reduce the risk of adverse outcomes, including preterm birth, low birth weight, and infant mortality. However, smoking cessation therapies also carry risks in pregnant women. Using NRTs during the early stages of pregnancy may increase the risk of birth defects, according to a study of 77,000 pregnant Danish women.40
Other pharmacologic aids have not been tested for safety and effectiveness in treating tobacco dependence in pregnant women. Therefore, pregnant smokers should be offered intensive person-to-person interventions that exceed minimal advice to quit, such as behavioral support and problem solving, counseling, and referral to support organizations.4
PERSONS WITH CORONARY HEART DISEASE
Smoking cessation substantially reduces the risk of coronary heart disease. The success rate of bupropion in patients with established cardiovascular disease is similar to that in healthy smokers,41 and the drug is recommended for smoking cessation in patients with cardiovascular disease. However, bupropion is cardiotoxic in overdose and should be considered as a cause of unexplained widening of the QRS complex on electrocardiography.42
Although varenicline improves one-year abstinence rates over placebo in smokers with cardiovascular disease, the U.S. Food and Drug Administration recently released a warning about a possible increase in cardiovascular risk with the drug. Although uncommon, increased rates of angina pectoris, nonfatal myocardial infarction, need for coronary revascularization, and peripheral vascular disease have been reported in patients taking varenicline.30
Data indicate that the benefits of NRTs for smoking cessation outweigh the risks of the therapies. However, NRTs may have the potential to trigger cardiac events in the immediate postmyocardial infarction period, in patients with serious cardiac arrhythmias, and in patients with serious or worsening angina pectoris.43 Physicians may consider a lower initial dosage, more frequent monitoring of side effects, and alternative therapies, such as behavioral interventions.
ADOLESCENTS
Ninety percent of adult smokers began smoking as adolescents or preadolescents.44 The U.S. Preventive Services Task Force found insufficient evidence to recommend tobacco screening or interventions in adolescents45; however, physicians may intervene with adolescent smokers using motivation-enhancing strategies such as the five A's and the five R's.4 Although considered safe for adolescents,46 NRTs may require more intensive instruction and lead to lower tobacco abstinence rates than in adults.
Data Sources: The following databases were searched: Essential Evidence Plus, PubMed, Cochrane Database of Systematic Reviews, National Guidelines Clearinghouse, U.S. Preventive Services Task Force, Dynamed, PEPID, and UpToDate. The search included meta-analyses, randomized controlled trials, clinical trials, guidelines, and reviews. The key term smoking cessation was searched with the other key terms interventions, therapies, medications, nicotine replacement, pregnancy, alternative, complementary, CAM, cardiovascular disease, and adolescents. Search dates: December 6, 2010, to March 1, 2011.
