
Am Fam Physician. 2012;85(6):654-655
Guideline source: American College of Obstetricians and Gynecologists
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Not reported
Published source: Obstetrics & Gynecology, August 2011
Breast cancer–related death can be reduced through effective screening, which typically includes imaging, clinical examination, and self-examination or awareness. However, there is controversy regarding the value of these screening techniques, the age at which they should be initiated and stopped, and how often they should be performed. The American College of Obstetricians and Gynecologists recommends using all three techniques to screen for breast cancer (Table 1).
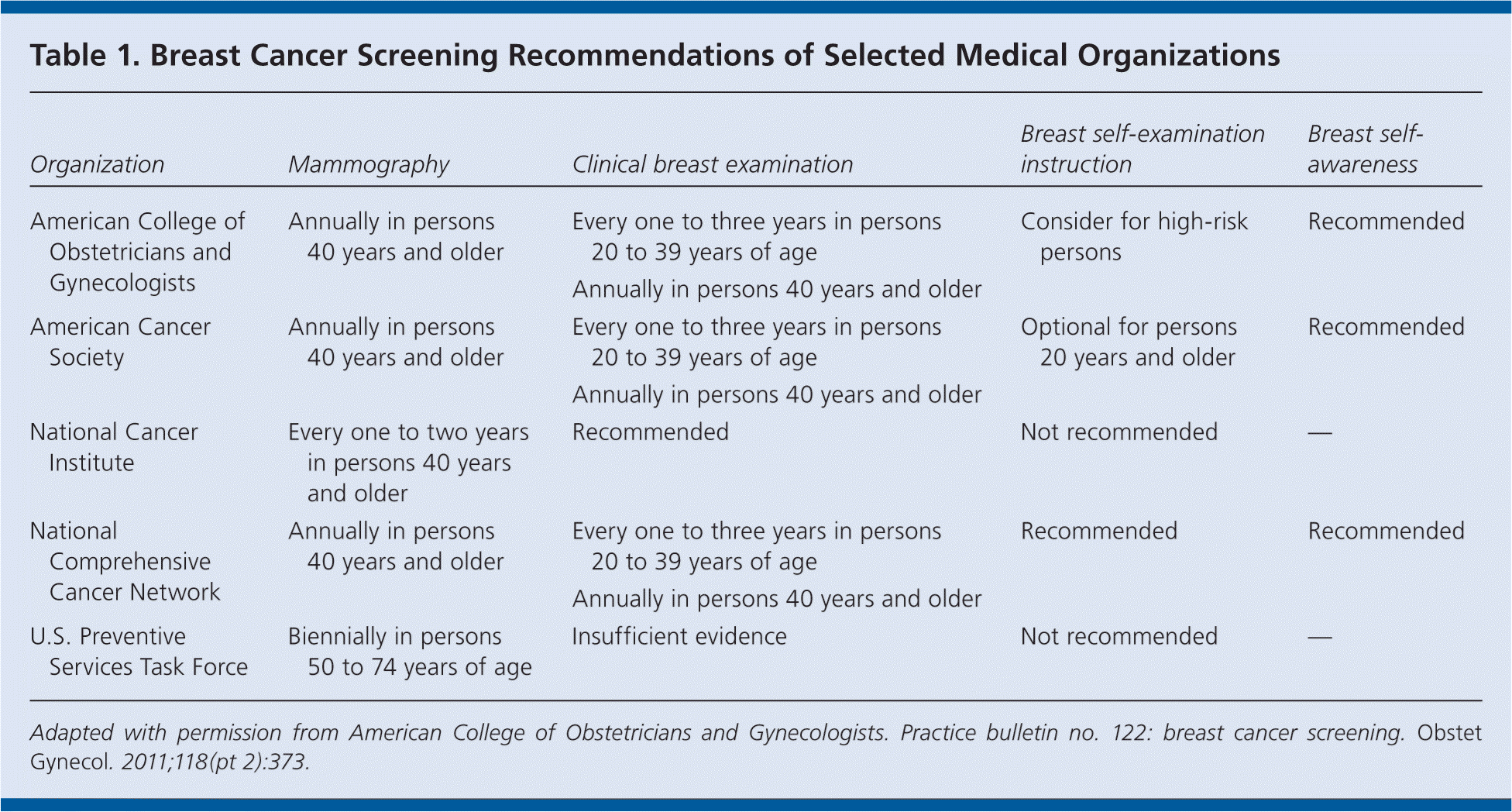
| Organization | Mammography | Clinical breast examination | Breast self-examination instruction | Breast self-awareness |
|---|---|---|---|---|
| American College of Obstetricians and Gynecologists | Annually in persons 40 years and older | Every one to three years in persons 20 to 39 years of age | Consider for high-risk persons | Recommended |
| Annually in persons 40 years and older | ||||
| American Cancer Society | Annually in persons 40 years and older | Every one to three years in persons 20 to 39 years of age | Optional for persons 20 years and older | Recommended |
| Annually in persons 40 years and older | ||||
| National Cancer Institute | Every one to two years in persons 40 years and older | Recommended | Not recommended | — |
| National Comprehensive Cancer Network | Annually in persons 40 years and older | Every one to three years in persons 20 to 39 years of age | Recommended | Recommended |
| Annually in persons 40 years and older | ||||
| U.S. Preventive Services Task Force | Biennially in persons 50 to 74 years of age | Insufficient evidence | Not recommended | — |
Recommendations
Women 20 years and older should be taught about breast self-awareness. Breast self-awareness is a woman's awareness of the normal feel and appearance of her breasts. The objective is to highlight the importance of self-detection and timely assessment of symptoms. The effects of breast self-awareness education have not yet been studied.
Clinical breast examination should be performed annually in women 40 years and older, and every one to three years in women 20 to 39 years of age. Reviews support combining clinical examination with mammography. The usefulness of clinical examination in women with a low prevalence of breast cancer (i.e., women 20 to 39 years of age) is not known.
Mammography should be performed annually in women 40 years and older. Education should be provided regarding the predictive value of mammography; the possibility of false-positive and false-negative results; and the possibility of needing more imaging or biopsies based on screening results. Decisions regarding screening should be based on individual risks and benefits. Some women may prefer annual screening because it maximizes cancer detection, whereas others may prefer biennial screening because it provides most of the benefits, but reduces the frequency of screening and possibility of requiring more testing. Table 2 lists the relative risks for breast cancer mortality from mammography screening trials.
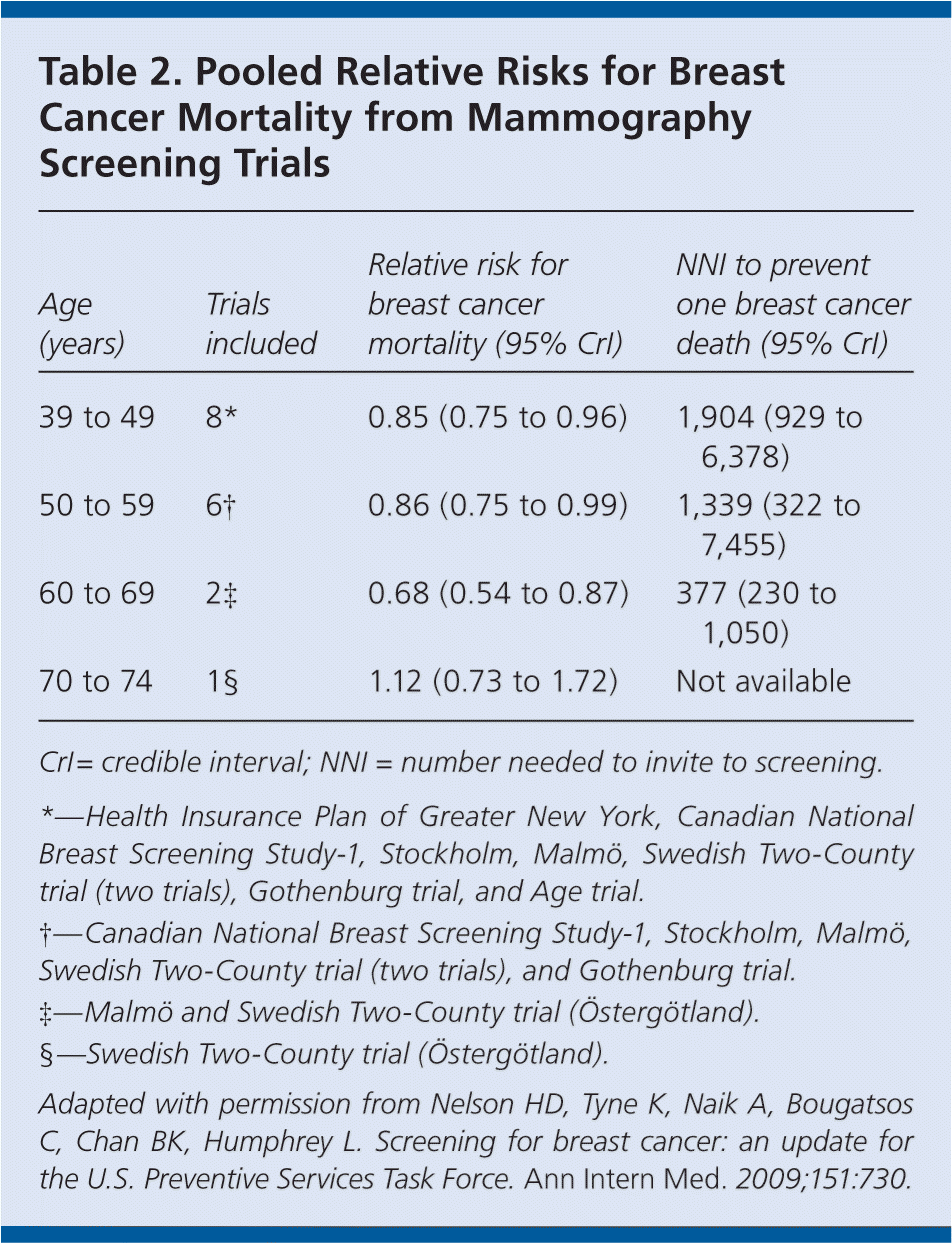
| Age (years) | Trials included | Relative risk for breast cancer mortality (95% CrI) | NNI to prevent one breast cancer death (95% CrI) |
|---|---|---|---|
| 39 to 49 | 8* | 0.85 (0.75 to 0.96) | 1,904 (929 to 6,378) |
| 50 to 59 | 6† | 0.86 (0.75 to 0.99) | 1,339 (322 to 7,455) |
| 60 to 69 | 2‡ | 0.68 (0.54 to 0.87) | 377 (230 to 1,050) |
| 70 to 74 | 1§ | 1.12 (0.73 to 1.72) | Not available |
Although most women with breast cancer do not have identifiable risk factors, those with certain characteristics have an increased prevalence of breast cancer compared with the general population. Physicians should regularly assess breast cancer risk to determine if a woman is at average, increased, or high risk, and to decide which women qualify for enhanced screening. Table 3 lists factors that increase the relative risk of breast cancer.

| Relative risk | Factor |
|---|---|
| > 4.0 | Age (65 years and older vs. younger than 65 years, although risk increases across all ages until 80 years of age) |
| Biopsy-confirmed atypical hyperplasia | |
| Certain inherited genetic mutations for breast cancer (BRCA1 and/or BRCA2) | |
| Mammographically dense breasts | |
| Personal history of breast cancer | |
| 2.1 to 4.0 | High bone density (postmenopausal) |
| High-dose radiation to chest | |
| High endogenous estrogen or testosterone levels | |
| Two first-degree relatives with breast cancer | |
| 1.1 to 2.0 | Alcohol consumption |
| Ashkenazi Jewish heritage | |
| Early menarche (younger than 12 years) | |
| Height (tall) | |
| High socioeconomic status | |
| Late age at first full-term pregnancy (older than 30 years) | |
| Late menopause (older than 55 years) | |
| Never breastfed a child | |
| No full-term pregnancies | |
| Obesity (postmenopausal)/adult weight gain | |
| One first-degree relative with breast cancer | |
| Personal history of endometrial, ovarian, or colon cancer | |
| Recent and long-term use of estrogen and progestin | |
| Recent oral contraceptive use |
Enhanced screening should be recommended, and risk reduction methods should be discussed, in women who test positive for BRCA1 or BRCA2 gene mutations. Women with an estimated lifetime breast cancer risk of at least 20 percent based on risk models that rely mostly on family history, but who have not been tested or who have tested negative for BRCA gene mutations can also be offered enhanced screening. Enhanced screening includes biannual clinical examination, annual mammography, annual magnetic resonance imaging, and guidance in self-examination. Women with a first-degree relative with these gene mutations, but who are untested themselves are typically treated as if they have the mutations.
Breast magnetic resonance imaging is not recommended in women at average risk of breast cancer. The American Cancer Society recommends magnetic resonance imaging in high-risk women (i.e., at least a 20 percent lifetime risk).
