
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2012;85(7):705-710
A more recent article on end-stage renal disease is available.
Patient information: See related handout on kidney failure, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
The prevalence of end-stage renal disease continues to increase, and dialysis is offered to older and more medically complex patients. Pain is problematic in up to one-half of patients receiving dialysis and may result from renal and nonrenal etiologies. Opioids can be prescribed safely, but the patient's renal function must be considered when selecting a drug and when determining the dosage. Fentanyl and methadone are considered the safest opioids for use in patients with end-stage renal disease. Nonpain symptoms are common and affect quality of life. Phosphate binders, ondansetron, and naltrexone can be helpful for pruritus. Fatigue can be managed with treatment of anemia and optimization of dialysis, but persistent fatigue should prompt screening for depression. Ondansetron, metoclopramide, and haloperidol are effective for uremia-associated nausea. Nondialytic management may be preferable to dialysis initiation in older patients and in those with additional life-limiting illnesses, and may not significantly decrease life expectancy. Delaying dialysis initiation is also an option. Patients with end-stage renal disease should have advance directives, including documentation of situations in which they would no longer want dialysis.
The number of patients with end-stage renal disease is increasing in the United States, in part because of the epidemic of diabetes mellitus. Dialysis is now offered to older and more medically complex patients who would not have been considered for treatment in the days of limited dialysis resources. The prevalence of coronary artery disease in patients receiving dialysis increased from 0.1 percent in 2004 to 21.0 percent in 2007; the prevalence of cancer and chronic obstructive pulmonary disease increased as well.1 Two-thirds of patients receiving dialysis have moderate or severe cognitive impairment.2
Symptom management and advance care planning are crucial for these patients. Family physicians may be called on to address advance directives or to support patients and families in decisions about dialysis initiation or withdrawal. In addition, family physicians must be prepared to manage symptoms associated with end-stage renal disease and adjust medication dosages appropriately.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Fentanyl and methadone are the preferred opioids for use in patients with end-stage renal disease. | C | 8, 10, 11 |
| Conservative (nondialytic) management of end-stage renal disease can be offered to older adults and patients with multiple comorbidities. | C | 23, 24, 26 |
| Delayed initiation of dialysis (when glomerular filtration rate is 5.0 to 7.0 mL per minute per 1.73 m2) yields equivalent outcomes to early initiation. | B | 27 |
| Patients with end-stage renal disease should have advance directives, including documentation of situations in which they would want to discontinue dialysis. | C | 31, 34 |
Symptom Management
End-stage renal disease is associated with a large symptom burden. In one recent study, patients receiving dialysis reported an average of nine symptoms that resulted in impaired quality of life.3 In a second study, symptom burden and quality of life in advanced renal failure were similar to those in terminal malignancy.4 Up to one-half of symptoms of end-stage renal disease go untreated.5 In light of these findings, the United Kingdom recently adopted national guidelines for symptom management in advanced chronic kidney disease.6 Formal recommendations for renal palliative care have been slower to evolve in the United States, and symptom management is often left to family physicians.
PAIN
Pain is extremely common in end-stage renal disease and can result from renal and nonrenal etiologies. In a prospective cohort study of 205 patients receiving hemodialysis, 50 percent reported a problem with pain.7 Musculoskeletal pain was most common (63.1 percent), followed by dialysis-associated pain (13.6 percent), peripheral neuropathy (12.6 percent), and peripheral vascular disease (9.7 percent).
Some causes of pain are specific to renal disease. Polycystic kidney disease can cause chronic abdominal pain. Secondary hyperparathyroidism often results in bone pain. Calciphylaxis is a relatively rare cause of severe generalized pain that occurs almost exclusively in patients receiving dialysis. Abnormal metabolism of calcium and phosphorus leads to vascular calcification and skin ischemia, which usually presents as a painful rash or wound but can rapidly progress to overt necrosis.8 Calciphylaxis is difficult to treat and may justify withdrawal of dialysis in favor of hospice care.
Pain management must take into account the likely etiology of pain as well as the patient's renal function and dialysis status. Some analgesics should be avoided, whereas the dosage of others must be adjusted. For mild pain, acetaminophen can be used safely without any dose adjustment.8 Nonsteroidal anti-inflammatory drugs (NSAIDs) should generally be avoided in patients with end-stage renal disease because uremia causes platelet dysfunction and increases the risk of gastrointestinal bleeding. NSAIDs should always be avoided in patients receiving peritoneal dialysis.8 In this type of dialysis, adequate solute clearance and maintenance of volume balance depends on small amounts of residual renal function, which may be threatened by NSAID use.9
For moderate to severe pain, tramadol (Ultram) can be used cautiously but requires dose adjustment and an increased dosing interval; the maximal dosage should not exceed 50 to 100 mg twice daily.10 Many patients will require opioid analgesics to achieve adequate pain control. Fentanyl and methadone are considered the safest opioids for use in patients with renal failure10,11; other opioids may be used with close monitoring and dose adjustment (Table 16,8,10,11 and Table 211,12 ).
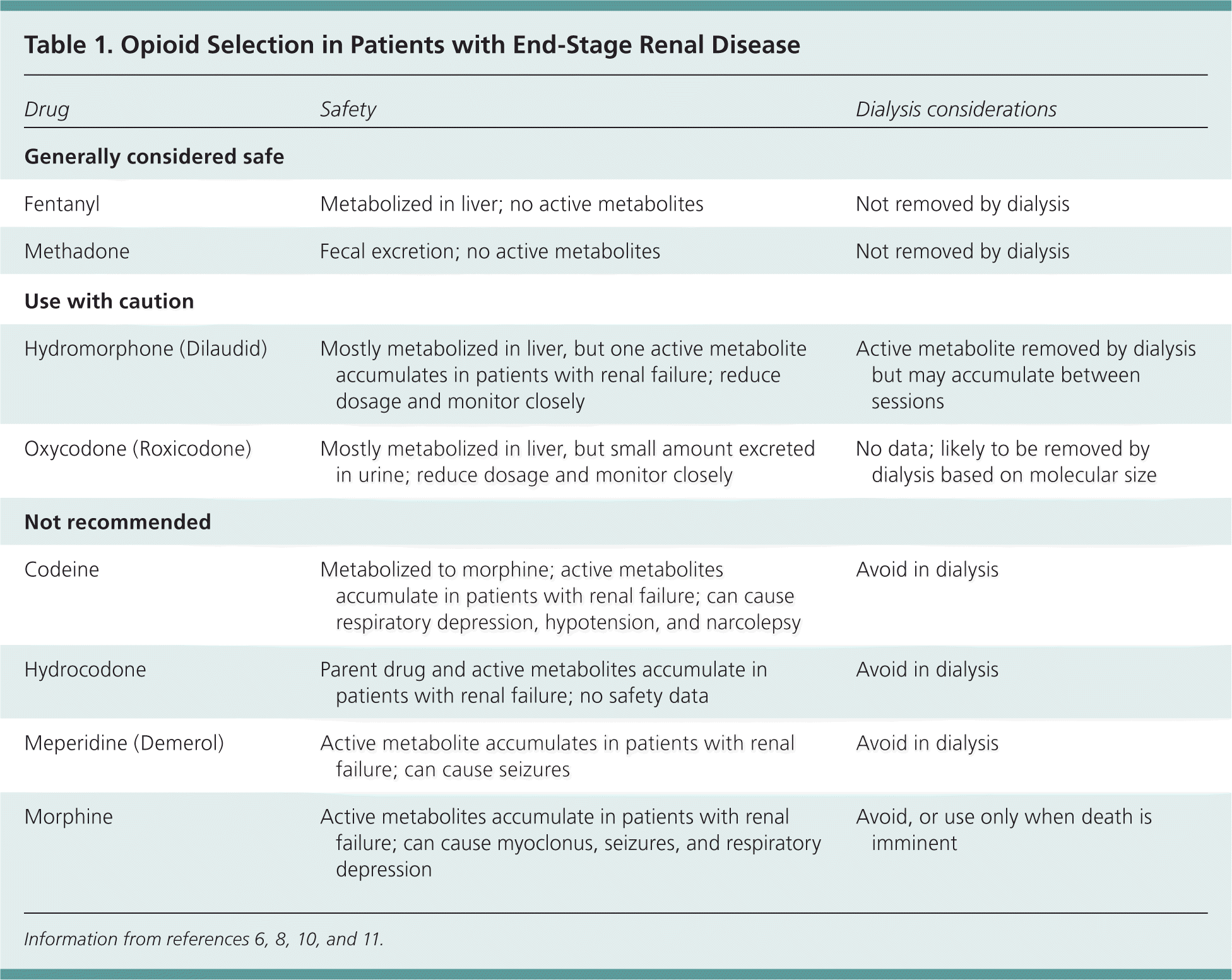
| Drug | Safety | Dialysis considerations |
|---|---|---|
| Generally considered safe | ||
| Fentanyl | Metabolized in liver; no active metabolites | Not removed by dialysis |
| Methadone | Fecal excretion; no active metabolites | Not removed by dialysis |
| Use with caution | ||
| Hydromorphone (Dilaudid) | Mostly metabolized in liver, but one active metabolite accumulates in patients with renal failure; reduce dosage and monitor closely | Active metabolite removed by dialysis but may accumulate between sessions |
| Oxycodone (Roxicodone) | Mostly metabolized in liver, but small amount excreted in urine; reduce dosage and monitor closely | No data; likely to be removed by dialysis based on molecular size |
| Not recommended | ||
| Codeine | Metabolized to morphine; active metabolites accumulate in patients with renal failure; can cause respiratory depression, hypotension, and narcolepsy | Avoid in dialysis |
| Hydrocodone | Parent drug and active metabolites accumulate in patients with renal failure; no safety data | Avoid in dialysis |
| Meperidine (Demerol) | Active metabolite accumulates in patients with renal failure; can cause seizures | Avoid in dialysis |
| Morphine | Active metabolites accumulate in patients with renal failure; can cause myoclonus, seizures, and respiratory depression | Avoid, or use only when death is imminent |
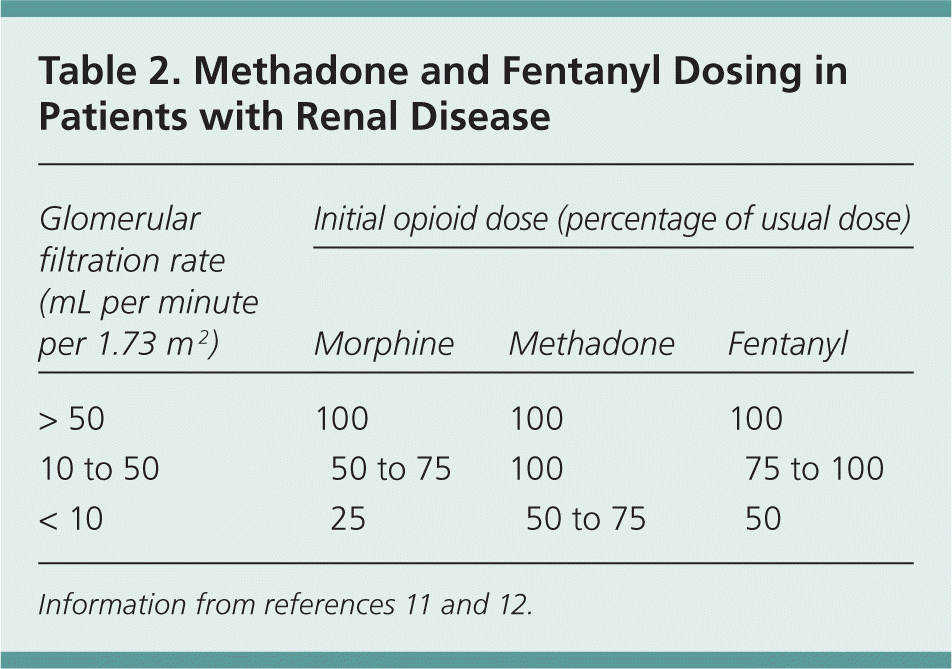
| Glomerular filtration rate(mL per minute per 1.73 m2 ) | Initial opioid dose (percentage of usual dose) | ||
|---|---|---|---|
| Morphine | Methadone | Fentanyl | |
| > 50 | 100 | 100 | 100 |
| 10 to 50 | 50 to 75 | 100 | 75 to 100 |
| < 10 | 25 | 50 to 75 | 50 |
Adjuvant medications are often added to augment opioids. Despite recent controversy about the strength of evidence for its use in neuropathic pain, gabapentin (Neurontin) is widely prescribed, and its dosage must be adjusted for renal function.13 Gabapentin is excreted unchanged in the urine; it can accumulate to potentially toxic levels in patients with renal failure. For patients receiving hemodialysis, a loading dose of 300 mg can be given, followed by 200 to 300 mg after each dialysis session.8 For patients who are not receiving dialysis but have creatinine clearance of less than 30 mL per minute per 1.73 m2, a dose of 200 to 700 mg can be given once daily.14 Tricyclic antidepressants are generally avoided in patients with chronic kidney disease; their proarrhythmic potential poses additional risk in the setting of fluctuating electrolyte abnormalities. Pregabalin (Lyrica) requires a decreased dosage and an increased dosing interval. Exact dosing depends on the indication and creatinine clearance.15
NONPAIN SYMPTOMS
Nonpain symptoms also contribute to decreased quality of life in patients with renal failure. Lack of energy and pruritus are reported by up to 75 percent of patients with stage 5 chronic kidney disease.16 More than one-half of patients have drowsiness, dyspnea, or edema16; additional symptoms include dry mouth, muscle cramps, restless legs syndrome, lack of appetite, poor concentration, sleep disturbance, and constipation. Many of these symptoms can be managed with simple interventions in the primary care setting (Table 3).6,8,17–20 [ corrected] Pruritus can be managed with phosphate binders, emollients, antihistamines, ondansetron (Zofran), and naltrexone (Revia).6,17 Fatigue can be managed by treating anemia, encouraging physical activity, and evaluating for depression.6,8 Ondansetron, metoclopramide (Reglan), and haloperidol are effective antiemetics for uremiaassociated nausea.6,19
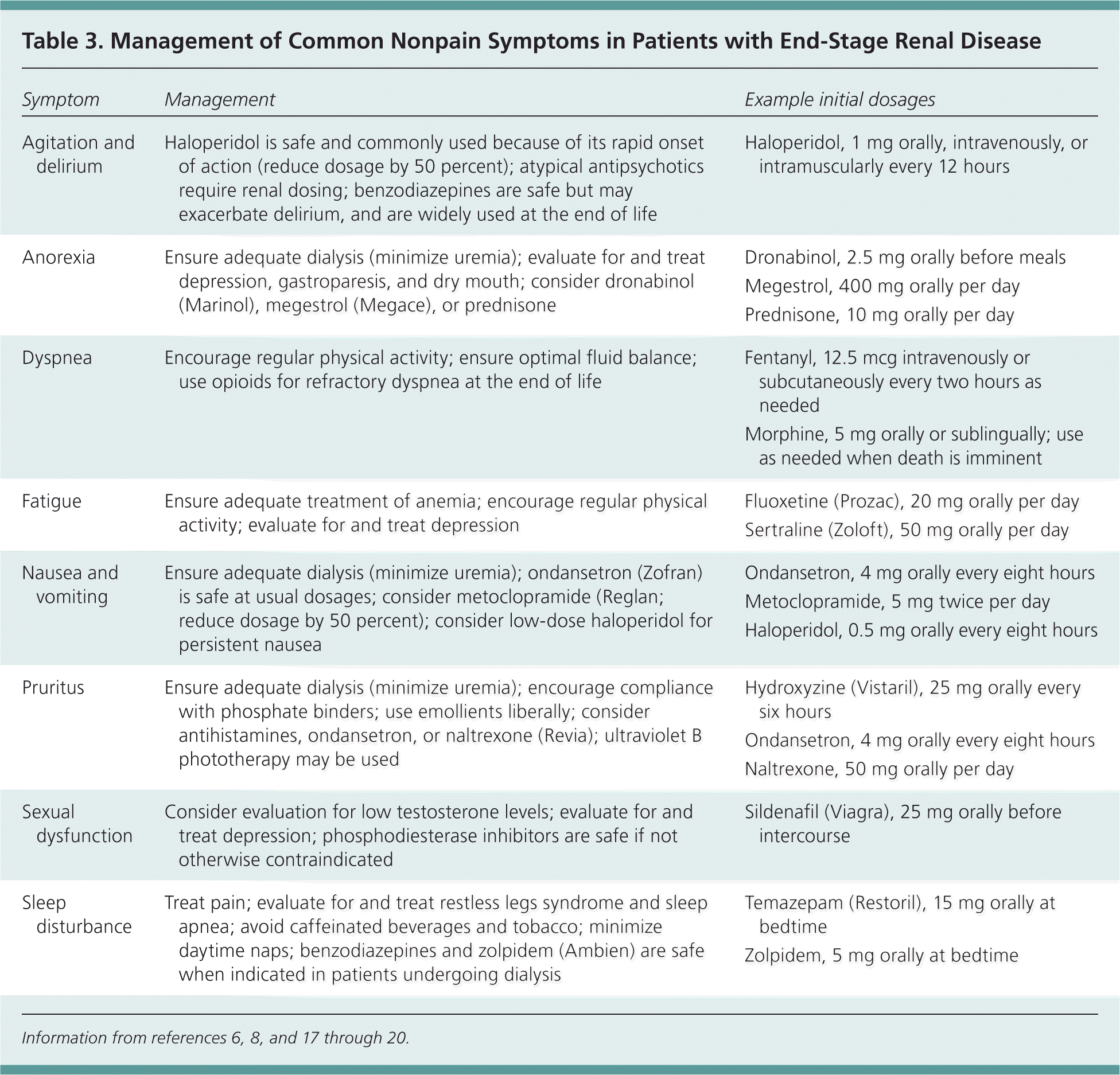
| Symptom | Management | Example initial dosages |
|---|---|---|
| Agitation and delirium | Haloperidol is safe and commonly used because of its rapid onset of action (reduce dosage by 50 percent); atypical antipsychotics require renal dosing; benzodiazepines are safe but may exacerbate delirium, and are widely used at the end of life | Haloperidol, 1 mg orally, intravenously, or intramuscularly every 12 hours |
| Anorexia | Ensure adequate dialysis (minimize uremia); evaluate for and treat depression, gastroparesis, and dry mouth; consider dronabinol (Marinol), megestrol (Megace), or prednisone | Dronabinol, 2.5 mg orally before meals |
| Megestrol, 400 mg orally per day | ||
| Prednisone, 10 mg orally per day | ||
| Dyspnea | Encourage regular physical activity; ensure optimal fluid balance; use opioids for refractory dyspnea at the end of life | Fentanyl, 12.5 mcg intravenously or subcutaneously every two hours as needed |
| Morphine, 5 mg orally or sublingually; use as needed when death is imminent | ||
| Fatigue | Ensure adequate treatment of anemia; encourage regular physical activity; evaluate for and treat depression | Fluoxetine (Prozac), 20 mg orally per day |
| Sertraline (Zoloft), 50 mg orally per day | ||
| Nausea and vomiting | Ensure adequate dialysis (minimize uremia); ondansetron (Zofran) is safe at usual dosages; consider metoclopramide (Reglan; reduce dosage by 50 percent); consider low-dose haloperidol for persistent nausea | Ondansetron, 4 mg orally every eight hours |
| Metoclopramide, 5 mg twice per day | ||
| Haloperidol, 0.5 mg orally every eight hours | ||
| Pruritus | Ensure adequate dialysis (minimize uremia); encourage compliance with phosphate binders; use emollients liberally; consider antihistamines, ondansetron, or naltrexone (Revia); ultraviolet B phototherapy may be used | Hydroxyzine (Vistaril), 25 mg orally every six hours |
| Ondansetron, 4 mg orally every eight hours | ||
| Naltrexone, 50 mg orally per day | ||
| Sexual dysfunction | Consider evaluation for low testosterone levels; evaluate for and treat depression; phosphodiesterase inhibitors are safe if not otherwise contraindicated | Sildenafil (Viagra), 25 mg orally before intercourse |
| Sleep disturbance | Treat pain; evaluate for and treat restless legs syndrome and sleep apnea; avoid caffeinated beverages and tobacco; minimize daytime naps; benzodiazepines and zolpidem (Ambien) are safe when indicated in patients undergoing dialysis | Temazepam (Restoril), 15 mg orally at bedtime |
| Zolpidem, 5 mg orally at bedtime |
Initiation of Dialysis
The decision to initiate dialysis is usually made by a nephrologist, but family physicians are ideally positioned to collaborate with nephrologists and counsel patients and families when decision making is difficult. This may be especially important when the patient is older or when there are additional life-limiting diseases.
CONSERVATIVE (NONDIALYTIC) MANAGEMENT
In response to the aging population and trends of dialyzing older and sicker patients, there has been growing interest in nondialytic alternatives for managing end-stage renal disease. The Renal Physicians Association and the American Society of Nephrology issued a practice guideline affirming the rights of patients to decline dialysis.21 Nondialytic management includes careful attention to fluid balance, treatment of anemia, and correction of acidosis and hyperkalemia. Blood pressure and metabolism of calcium and phosphorus must also be monitored. There is emerging evidence that dietary modifications may be helpful in prolonging life and decreasing symptoms.22
Patients, families, and some medical professionals may erroneously think that choosing not to start dialysis is equivalent to stopping dialysis. Although patients who discontinue dialysis die within one to two weeks, patients who decline dialysis initiation can live for months to years. Studies have showed that patients who refuse dialysis have a median life expectancy of 6.3 to 23.4 months.23,24 Functional status generally remains stable until the last month of life.25 Several studies have found little or no survival benefit with dialysis versus conservative management in older patients.23,26 Any modest survival benefit from dialysis decreases with the presence of comorbid conditions, especially ischemic heart disease.26 Family physicians can counsel older patients and those with multiple comorbidities that nondialytic management is a viable option in end-stage renal disease.
DELAYED INITIATION
Considerable clinical variation exists in the timing of dialysis initiation. A recent trial randomized 828 adults to early initiation of dialysis (at a glomerular filtration rate of 10.0 to 14.0 mL per minute per 1.73 m2) or late initiation (at 5.0 to 7.0 mL per minute per 1.73 m2).27
In three years of follow-up, there were no differences between groups in mortality, cardiovascular events, infections, or dialysis complications. This trial suggests that delayed dialysis initiation is an acceptable option, especially in patients who are unsure that they want dialysis. Delayed initiation may also be practical in patients for whom dialysis would be a significant hardship, such as those who work or have limited social support.
Advance Care Planning
The five-year survival rate for patients with stage 5 chronic kidney disease is 38 percent, less than that of AIDS and many cancers.28 For patients older than 65 years, the five-year survival rate is only 18 percent.1 Given this high mortality, advance care planning is a critical topic to address at all opportunities, such as annual physicals, hospital admissions, and routine office visits.
CARDIOPULMONARY RESUSCITATION
Patients with renal failure have poor outcomes after cardiopulmonary resuscitation (CPR). In one study of 74 patients receiving dialysis who underwent CPR for cardiopulmonary arrest, only 8 percent survived to hospital discharge, and only 3 percent were alive six months after CPR.29 These outcomes were significantly worse than those in patients who were not receiving dialysis (12 percent survival to hospital discharge, 9 percent survival six months after CPR). Patients who survive CPR often have neurologic compromise or need ongoing mechanical ventilation.
Despite these statistics, many patients with end-stage renal disease have unrealistically optimistic expectations of CPR. In one study, 87 percent of patients receiving dialysis reported that they would want CPR in the event of cardiopulmonary arrest.30 Blacks were significantly more likely to want CPR than whites (adjusted odds ratio = 6.56, 95% confidence interval, 2.57 to 22.27). Family physicians can help educate patients about the low likelihood of successful CPR in the setting of end-stage renal disease. They can also document do-not-resuscitate status if necessary.
ADVANCE DIRECTIVES
Advance directives (i.e., a living will and durable power of attorney for health care) can guide physicians in end-of-life decision making. In addition, patients who complete written advance directives are more likely to informally discuss their wishes for specific medical interventions with their families.31 As many as one-half of patients who receive dialysis do not have advance directives.32 One study showed that less than 10 percent of patients had discussions about end-of-life care with their physicians, and 61 percent reported that they regretted their decision to start dialysis.33
Most advance directives do not describe situations in which a patient would no longer want dialysis. Withdrawal of dialysis is common in end-stage renal disease; therefore, disease-specific advance directives that include dialysis withdrawal have been proposed.34 Family physicians can facilitate preemptive conversations about these issues with patients and their decision makers.
HOSPICE ELIGIBILITY
Patients whose only life-limiting diagnosis is end-stage renal disease are not eligible for the Medicare hospice benefit while they are receiving dialysis. If patients have another terminal diagnosis, they may receive concurrent hospice and dialysis benefits under Medicare. For example, a patient receiving dialysis who develops metastatic cancer may continue dialysis while receiving hospice services for cancer.35 Patients who stop dialysis are always eligible for hospice care and should be routinely referred. However, only 41.9 percent of patients who withdrew from dialysis in 2001 and 2002 received hospice services.36 Hospice care decreases the cost of care and increases the likelihood of dying at home after dialysis withdrawal.36
Data Sources: A Medline search was conducted using the key terms end-stage renal disease and kidney failure, chronic. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. The results were then combined with the following search terms: pain, palliative care, advance care planning, quality of life, and terminal care. Also searched were the Cochrane Database, the National Guideline Clearinghouse, Essential Evidence Plus, and Fast Facts for Palliative Care. Search date: January 28, 2011.
