
A more recent article on infective endocarditis is available.
Am Fam Physician. 2012;85(10):981-986
Author disclosure: No relevant financial affiliations to disclose.
Infectious endocarditis results from bacterial or fungal infection of the endocardial surface of the heart and is associated with significant morbidity and mortality. Risk factors include the presence of a prosthetic heart valve, structural or congenital heart disease, intravenous drug use, and a recent history of invasive procedures. Endocarditis should be suspected in patients with unexplained fevers, night sweats, or signs of systemic illness. Diagnosis is made using the Duke criteria, which include clinical, laboratory, and echocardiographic findings. Antibiotic treatment of infectious endocarditis depends on whether the involved valve is native or prosthetic, as well as the causative microorganism and its antibiotic susceptibilities. Common blood culture isolates include Staphylococcus aureus, viridans Streptococcus, enterococci, and coagulase-negative staphylococci. Valvular structural and functional integrity may be adversely affected in infectious endocarditis, and surgical consultation is warranted in patients with aggressive or persistent infections, emboli, and valvular compromise or rupture. After completion of antibiotic therapy, patients should be educated about the importance of daily dental hygiene, regular visits to the dentist, and the need for antibiotic prophylaxis before certain procedures.
The incidence of endocarditis is approximately 5 to 7.9 cases per 100,000 persons per year in the United States,1 and has been stable over time. Risk factors for infectious endocarditis include hemodialysis (7.9 percent), intravenous drug use (9.8 percent), degenerative valvular disease (mitral regurgitation in 43.4 percent; aortic regurgitation in 26.3 percent), and rheumatic heart disease (3.3 percent).2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Infectious endocarditis should be suspected in patients who have unexplained fevers, particularly in the presence of risk factors or cardiac findings. | C | 1 |
| Initial empiric therapy in patients with suspected endocarditis should include vancomycin or ampicillin/sulbactam (Unasyn) plus an aminoglycoside (plus rifampin in patients with prosthetic valves). | B | 1 |
| Valve replacement should be considered in selected patients with infectious endocarditis. | B | 18 |
| Patients who have been successfully treated for infectious endocarditis in the past require antimicrobial prophylaxis before certain dental and other procedures. | C | 19 |
The International Collaboration on Endocarditis was formed in 1999; it consists of 58 hospitals in 25 countries. From 2000 to 2005, it studied 2,781 consecutive cases of endocarditis as defined by the modified Duke criteria.2 The median age of affected patients was 57.9 years, and 72.1 percent had endocarditis of the native valve.
Pathophysiology
The development of infectious endocarditis requires the presence of bacteria or fungi in the blood and an intracardiac surface on which these microorganisms can attach. Mechanical and biomechanical prosthetic heart valves can serve as foci for platelet adhesion and thrombus formation. These sites in turn provide extra surface area to which microorganisms can adhere and form vegetations3 (Figure 1).
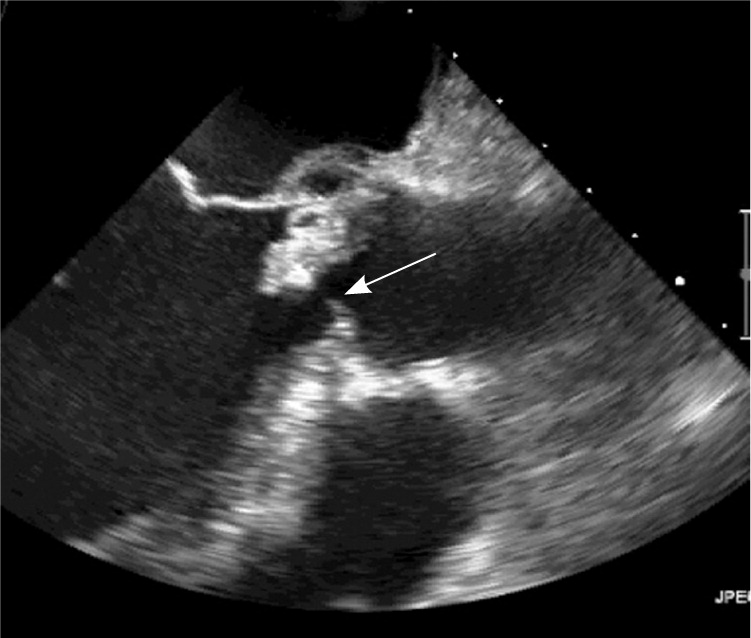
Early infection, which occurs within two months of valve placement, is generally the result of intraoperative contamination of the prosthesis or of postoperative infection. Late infection, which occurs at least 12 months after placement of the prosthesis, involves microbes and entry portals similar to those of native valve endocarditis.3 Late prosthetic valve endocarditis can also cause perivalvular invasion and extension into nearby tissue, potentially evolving into myocardial abscess, pericarditis, or conduction system disruption and heart block.
Diagnosis
Endocarditis should be suspected in any patient with unexplained fevers, night sweats, or signs of systemic illness, particularly if any of the following risk factors are present1: a prosthetic heart valve, structural or congenital heart disease, intravenous drug use, and a recent history of invasive procedures (e.g., wound care, hemodialysis). Clinical history consistent with infectious endocarditis includes the combination of a prior cardiac lesion and evidence of a recent source of bacteremia.
The diagnosis of infectious endocarditis requires multiple clinical, laboratory, and imaging findings. Overdiagnosis and underdiagnosis of infectious endocarditis can be problematic; a missed diagnosis could prove fatal, whereas overdiagnosis can result in weeks of unnecessary antibiotic treatment.
The widely accepted Duke criteria use a set of major and minor clinical and pathologic criteria to classify infectious endocarditis as definite, possible, or rejected (Table 1).4 Direct evidence of endocarditis can be obtained from histologic specimens collected during surgery or autopsy, or from a combination of two major clinical criteria, one major and three minor criteria, or five minor criteria. Possible endocarditis is defined as the presence of one major and one or two minor criteria, or three minor criteria.4

| Major criteria | ||
| Positive blood culture | ||
| Two separate blood cultures positive for microorganism consistent with infectious endocarditis (viridans Streptococcus, Streptococcus bovis, gram-negative HACEK bacilli, Staphylococcus aureus, or community-acquired enterococci in the absence of a primary focus) | ||
| or | ||
| Recovery of a microorganism consistent with infectious endocarditis from blood cultures drawn more than 12 hours apart | ||
| or | ||
| Recovery of a microorganism consistent with infectious endocarditis from all of three or most of four or more blood cultures, with first and last drawn more than one hour apart | ||
| or | ||
| Single positive blood culture for Coxiella burnetii or phase 1 immunoglobulin G antibody titer greater than 1:800 | ||
| Evidence of endocardial involvement | ||
| Positive echocardiography (oscillating intracardiac mass on valve or supporting structures, or in the path of regurgitant jets, or on implanted material in the absence of an alternative anatomic explanation; intracardiac abscess; new partial dehiscence of prosthetic valve) | ||
| New valvular regurgitation (increase or change in preexisting murmur not sufficient) | ||
| Minor criteria | ||
| Fever of at least 38.0°C (100.4°F) | ||
| Immunologic phenomena: glomerulonephritis, Osler nodes, Roth spots, rheumatoid factor | ||
| Microbiologic evidence: positive blood culture that does not meet major criteria, serologic evidence of active infection with organism consistent with infectious endocarditis | ||
| Predisposing heart condition or history of injection drug use | ||
| Vascular phenomena: major arterial emboli, septic pulmonary infarctions, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, Janeway lesions | ||
Clinical Presentation
Preexisting structural abnormalities of the heart are present in 75 percent of patients with infectious endocarditis.5 Historically, rheumatic heart disease was the most common cardiac abnormality in infectious endocarditis6; however, degenerative lesions such as mitral valve prolapse are becoming an increasingly prevalent cause.5 Aortic valve disease and congenital heart disease in the setting of bacteremia are also common risk factors.
Fewer than one-half of persons with infectious endocarditis who use injection drugs have evidence of a structural or congenital valvular lesion, with estimates between 6 and 40 percent.7,8 Instead, injection of microorganisms or particulate matter from the skin itself or from within the drug material may generate transient or permanent endothelial damage to the tricuspid valve, thus providing an area for vegetations to develop.7 In addition, particulates smaller than 10 micrometers may cross pulmonary capillaries and damage surfaces of the aortic and mitral valves.9 In general, right-sided infectious endocarditis is far less common than left-sided, and most cases occur on the tricuspid valve in persons who use injection drugs.8 Pulmonic valve involvement is rare. Tricuspid valve endocarditis does not usually result in any detectable murmur,7 which complicates diagnosis.
Endocarditis in persons who use injection drugs is likely to be right-sided; therefore, septic pulmonary emboli are common, whereas manifestations of endocarditis (e.g., splinter and conjunctival hemorrhages) are less likely.10 Because blood cultures in these patients are usually positive, it is appropriate to draw blood in febrile patients and consider starting empiric antibiotics, depending on the clinical severity of illness.9,10
Nosocomial infectious endocarditis is defined as a new diagnosis of infectious endocarditis made three to 60 days after admission to a hospital or long-term care unit, during which there was risk of bacteremia. It is generally a complication of bacteremia introduced by an invasive procedure or indwelling catheter.11 In some areas, up to 20 percent of infectious endocarditis cases are nosocomial.12 Patients receiving chronic hemodialysis are also at risk of developing infectious endocarditis because of chronic intravenous access, immune system impairment, and calcific valvular disease.13
Evaluation
Physical examination should focus on cardiac auscultation for signs of a new regurgitant murmur or heart failure, as well as classical clinical stigmata of endocarditis, such as petechiae of the mucous membranes, retina (e.g., Roth spots [retinal hemorrhages with pale centers]), or extremities (e.g., splinter hemorrhages, Janeway lesions [macular or nodular hemorrhagic lesions on the palms or soles], Osler nodes [painful raised lesions on the palms and soles]).4
Blood cultures should be obtained before initiation of antibiotic therapy.14 In critically ill patients, a minimum of three cultures from different venipuncture sites should be drawn over one hour before starting empiric therapy.3 In non–critically ill patients in whom endocarditis is suspected, therapy may be delayed until the results of blood cultures and echocardiography are available. Obtaining more than three blood cultures typically yields only minimal additional diagnostic information.15
Other laboratory findings, such as elevated erythrocyte sedimentation rate and C-reactive protein levels, are relatively nonspecific3; urinalysis may show evidence of gross or microscopic hematuria, proteinuria, or pyuria caused by the immunologic effects of endocarditis on the kidneys. White blood cell count may be normal or elevated.
Baseline electrocardiography should be performed in patients with infectious endocarditis so that new cardiac manifestations can be recognized early (e.g., extension of valvular disease into the conduction system, ischemia secondary to emboli to the coronary circulation).3 If tricuspid valve endocarditis is suspected in persons who use injection drugs, chest radiography may reveal evidence of septic pulmonary emboli.
The American College of Cardiology and the American Heart Association recommend that echocardiography be performed to identify valvular abnormalities in all patients in whom there is moderate or high suspicion of endocarditis.16 Transthoracic echocardiography is usually the initial imaging modality. However, transesophageal echocardiography may be necessary in some patients, such as those with staphylococcus bacteremia, limited transthoracic windows because of obesity or mechanical ventilation, a prosthetic valve that renders visualization difficult secondary to shadowing, a history of endocarditis, or a structural valve abnormality.
Treatment
ANTIBIOTICS
Successful treatment requires appropriate antibiotic therapy. Initial empiric therapy may include vancomycin or ampicillin/sulbactam (Unasyn) plus an aminoglycoside (plus rifampin in patients with prosthetic valves).1 The choice of definitive antibiotic therapy is based on the causative microorganism and its antibiotic susceptibility, and whether the involved valve is native or prosthetic. Table 2 shows the incidence of various microorganisms identified in a long-term multicenter study of infectious endocarditis.2 Table 3 summarizes antibiotic recommendations from the American Heart Association.16
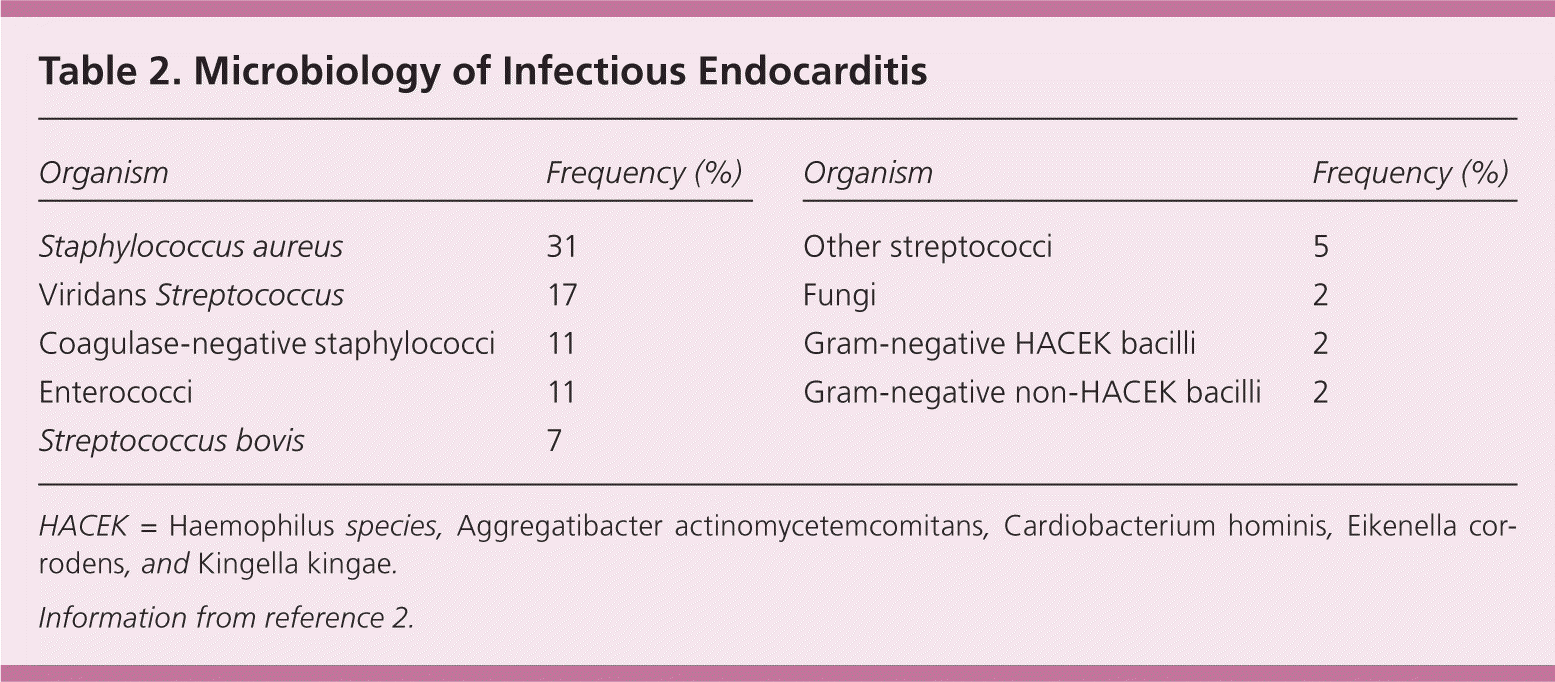
| Organism | Frequency (%) | |
|---|---|---|
| Staphylococcus aureus | 31 | |
| Viridans Streptococcus | 17 | |
| Coagulase-negative staphylococci | 11 | |
| Enterococci | 11 | |
| Streptococcus bovis | 7 | |
| Other streptococci | 5 | |
| Fungi | 2 | |
| Gram-negative HACEK bacilli | 2 | |
| Gram-negative non-HACEK bacilli | 2 | |

| Microorganism | Parenteral antibiotic regimen | |
|---|---|---|
| Penicillin-susceptible viridans Streptococcus or Streptococcus bovis | Penicillin G or ceftriaxone (Rocephin) for four weeks | |
| or | ||
| Penicillin G plus gentamicin for two weeks | ||
| or | ||
| Ceftriaxone plus gentamicin for two weeks | ||
| or | ||
| Vancomycin for four weeks | ||
| Relatively penicillin-resistant viridans Streptococcus or S. bovis | Penicillin G or ceftriaxone for four weeks, plus gentamicin for two weeks | |
| or | ||
| Vancomycin for four weeks | ||
| Penicillin-resistant viridans Streptococcus or S. bovis | Ampicillin plus gentamicin for four to six weeks | |
| or | ||
| Penicillin G plus gentamicin for four to six weeks | ||
| or | ||
| Vancomycin for six weeks | ||
| Oxacillin-susceptible staphylococci | Nafcillin or oxacillin for six weeks, plus gentamicin for three to five days (optional) | |
| or | ||
| Cefazolin for six weeks, plus gentamicin for three to five days (optional) | ||
| Oxacillin-resistant staphylococci | Vancomycin for six weeks | |
| Enterococcus strains susceptible to penicillin, gentamicin, and vancomycin | Ampicillin plus gentamicin for four to six weeks | |
| or | ||
| Penicillin plus gentamicin for four to six weeks | ||
| or | ||
| Vancomycin and gentamicin for six weeks | ||
| Enterococcus strains susceptible to penicillin, streptomycin, and vancomycin, and resistant to gentamicin | Ampicillin or penicillin plus streptomycin for four to six weeks | |
| or | ||
| Vancomycin plus streptomycin for six weeks | ||
| Enterococcus strains resistant to penicillin, but susceptible to aminoglycosides and vancomycin | Ampicillin/sulbactam (Unasyn) plus gentamicin for a minimum of six weeks | |
| or | ||
| Vancomycin plus gentamicin for six weeks | ||
For the purposes of determining duration of therapy, the first day in which negative blood cultures are obtained is considered the first day of therapy. At least two sets of blood cultures should be obtained every 24 to 48 hours until the infection has cleared the bloodstream.17
SURGERY
The structural and functional integrity of cardiac valves may be damaged by infection.7 This may lead to valvular regurgitation or flow obstruction in valves with large vegetations.7 Surgery may need to be considered in selected patients; the benefits are greatest in patients with the most indications.18 Surgical intervention should be considered in patients with fungal infection, infection with aggressive antibiotic-resistant bacteria or bacteria that respond poorly to antibiotics, left-sided infectious endocarditis caused by gram-negative bacteria, persistent infection with positive blood cultures after one week of antibiotic therapy, or one or more embolic events during the first two weeks of antibiotic therapy.17 Surgical intervention is warranted for valve dehiscence, perforation, rupture or fistula, or a large perivalvular abscess.17 Periannular extension of infection into the myocardium is associated with increased mortality and should be suspected in patients presenting with new atrioventricular block.7
ANTICOAGULATION
Anticoagulation in patients with infectious endocarditis is controversial, particularly in those with mechanical valve endocarditis. In general, anticoagulation should be discontinued for at least the first two weeks of antibiotic therapy in patients with Staphylococcus aureus prosthetic valve endocarditis who have experienced a recent central nervous system embolic event.3
FOLLOW-UP AND PATIENT EDUCATION
Intravenous catheters should be removed promptly after antibiotic therapy is complete. Transthoracic echocardiography should be performed to establish a new baseline. In patients with a history of infectious endocarditis, three sets of blood cultures should be obtained from separate sites before antibiotics are initiated for febrile illness.
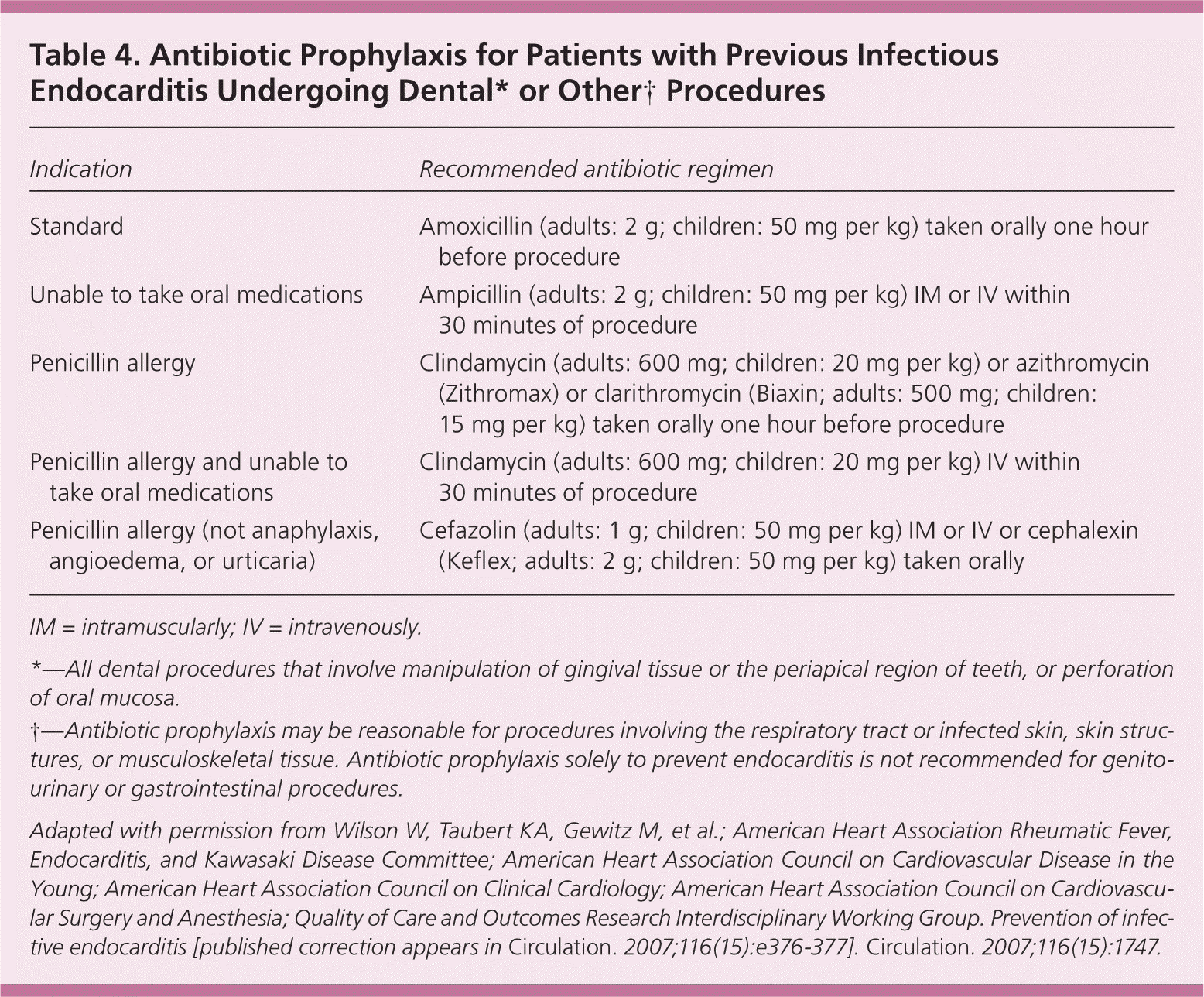
| Indication | Recommended antibiotic regimen |
|---|---|
| Standard | Amoxicillin (adults: 2 g; children: 50 mg per kg) taken orally one hour before procedure |
| Unable to take oral medications | Ampicillin (adults: 2 g; children: 50 mg per kg) IM or IV within 30 minutes of procedure |
| Penicillin allergy | Clindamycin (adults: 600 mg; children: 20 mg per kg) or azithromycin (Zithromax) or clarithromycin (Biaxin; adults: 500 mg; children: 15 mg per kg) taken orally one hour before procedure |
| Penicillin allergy and unable to take oral medications | Clindamycin (adults: 600 mg; children: 20 mg per kg) IV within 30 minutes of procedure |
| Penicillin allergy (not anaphylaxis, angioedema, or urticaria) | Cefazolin (adults: 1 g; children: 50 mg per kg) IM or IV or cephalexin (Keflex; adults: 2 g; children: 50 mg per kg) taken orally |
