
Am Fam Physician. 2012;85(12):1134-1141
Author disclosure: No relevant financial affiliations to disclose.
The U.S. population is aging, and an increasing number of persons are taking bisphosphonates for osteoporosis. National data on prescribing patterns show that in 2003, bisphosphonates were prescribed at 73 percent of physician visits for osteoporosis.1 In 2007, more than 5.4 million patients in the United States were taking oral bisphosphonates.2 However, bisphosphonates are under scrutiny because of the risk of bisphosphonate-related osteonecrosis of the jaw. The American and Canadian Associations of Oral and Maxillofacial Surgeons have adopted a working definition of this condition. It is defined as exposed bone in the maxillofacial region that has persisted beyond eight weeks in a patient currently or previously treated with a bisphosphonate, and in the absence of a history of radiotherapy to the jaw.3,4
Bisphosphonates are prescribed orally and intravenously, with most patients who have osteoporosis taking low-dose oral therapy. On average, patients take oral bisphosphonates for 4.6 years (and a minimum of three years) before developing osteonecrosis of the jaw.5 The prevalence in patients taking oral bisphosphonates is very low, ranging from 0.07 to 0.10 percent,2 although it is considerably higher in patients with cancer who are taking high-dose intravenous bisphosphonates.3
Osteonecrosis related to bisphosphonates occurs almost exclusively in the jaw. Lesions can range in size and severity (Figures 1 through 3). The etiology is unclear. The jaw is an area of high bone turnover because of the presence of teeth and daily bone remodeling around the periodontal ligaments.6 The alveolar bone has a turnover rate 10 times that of the long bone.7 In the process of bone remodeling and turnover, old bones are resorbed by osteoclasts and new bones are formed by osteoblasts. Bisphosphonates bind to osteoclasts and accumulate at sites of high bone turnover. Hence, there is a higher concentration of bisphosphonates in the jaw. Some theories suggest, and there is some supporting evidence, that the necrosis is caused by the inhibition of bone remodeling secondary to the suppression of bone resorption.6,8,9
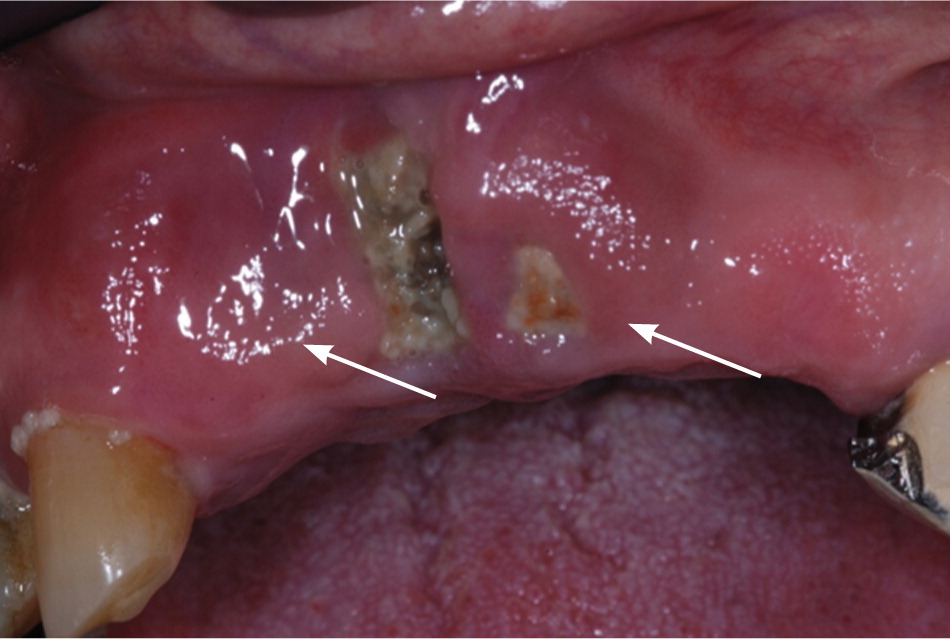
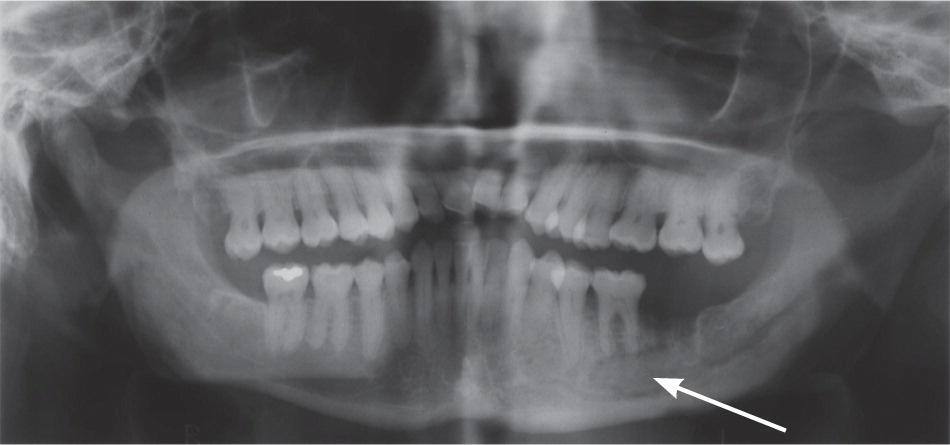
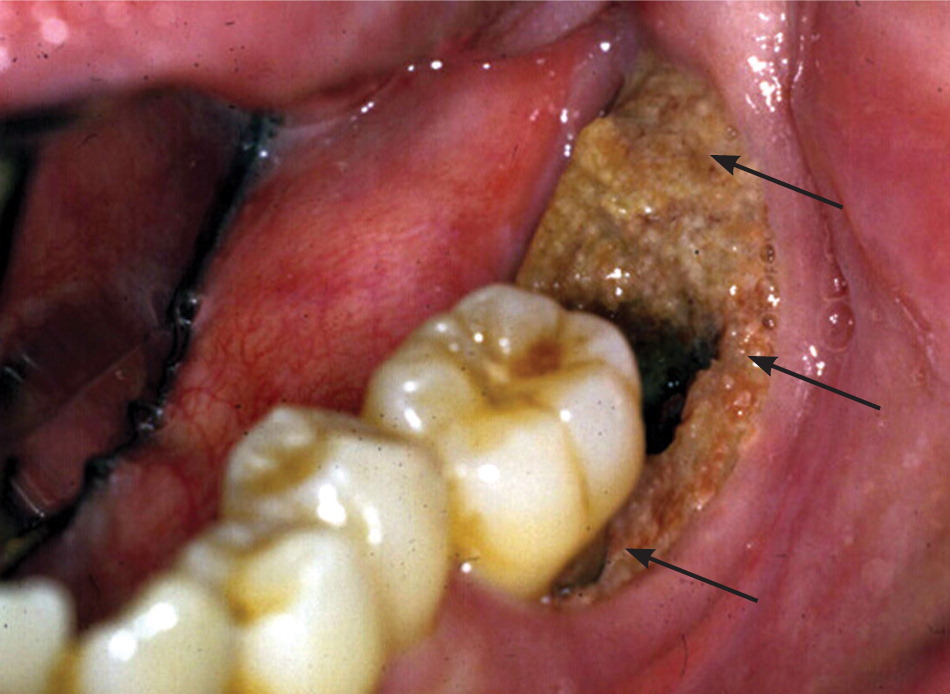
Dentoalveolar surgery, particularly tooth extraction, increases the risk of bisphosphonate-related osteonecrosis of the jaw by several-fold.3,10 Approximately 50 to 60 percent of cases were preceded by dentoalveolar surgery.7,8 Other contributing factors include local infection, corticosteroid use, and chemotherapy. It has been argued that the jaw is particularly vulnerable to infection compared with other bones because of the thin oral mucosa and high oral microbial load. In addition, bacteria have an easier path to the jaw via the thin periodontal ligaments.8 Bisphosphonates and other antiangiogenic drugs (e.g., glucocorticoids), and hypocellularity from chemotherapy impair bone healing and increase the risk of osteonecrosis.4,8,10
There are uncertainties concerning risk management and prevention of osteonecrosis of the jaw. Physicians prescribing bisphosphonates should advise patients about the small risk of osteonecrosis developing in the oral cavity. Physicians also should request or encourage a dental examination before the initiation of bisphosphonate therapy or shortly after starting treatment. Maintaining good oral health minimizes the risk.4,9,10
Currently, guidelines from the American Association of Oral and Maxillofacial Surgeons do not include a recommendation for the prevention of osteonecrosis of the jaw in patients taking oral bisphosphonates for less than three years and in the absence of other systemic risk factors (e.g., glucocorticoid therapy).3 However, for patients taking oral bisphosphonates for three years or more and for those with systemic risk factors, a drug holiday of at least three months before dentoalveolar surgery and throughout the healing period is recommended, if the underlying systemic disease is stable.3,4
Opponents of a drug holiday argue that, given the long half-life of bisphosphonates (i.e., up to 12 years), a drug holiday of a few months or even one year would do little to reduce the amount of bisphosphonates bound in bones.6,8,9 Nevertheless, there are reports of spontaneous healing of osteonecrotic sites and improved outcomes with cessation of bisphosphonate therapy.3,4,7,8,10 Therefore, a drug holiday may be beneficial for prevention and treatment.3,4
Some investigators have proposed measuring levels of serum C-terminal collagen peptides to stratify patients' risk of jaw osteonecrosis before dentoalveolar surgery.7 C-terminal collagen peptides are a biomarker of bone turnover, and bisphosphonates are known to suppress levels of C-terminal collagen peptides. Values less than 100 pg per mL are considered high risk, whereas values greater than 150 pg per mL represent minimal risk.7 The American Dental Association has questioned the usefulness of these measurements because there is too much variability (i.e., the normal range is wide) to make them meaningful.9
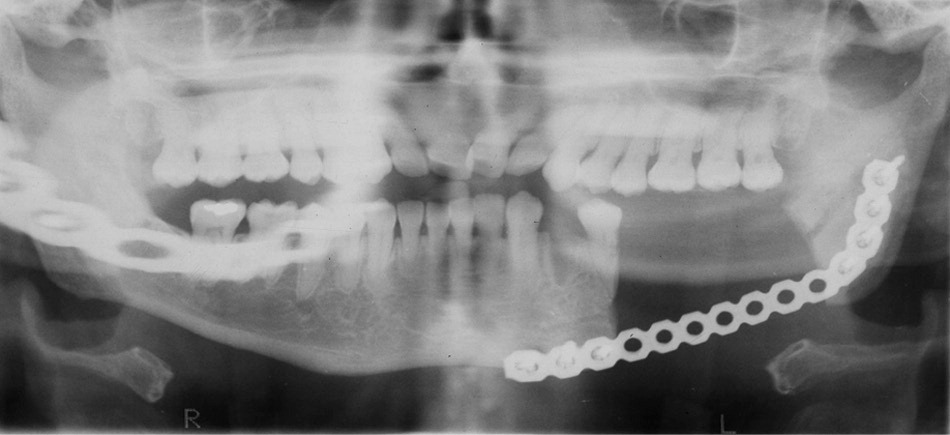
There is a paucity of evidence-based recommendations for the treatment of bisphosphonate-related osteonecrosis of the jaw because treatment outcome is relatively unpredictable.11 The microorganism commonly found in necrotic bones is Actinomyces.6,8,10 Antibiotic treatment for three weeks or longer has been suggested in combination with an antimicrobial oral rinse (e.g., chlorhexidine [Peridex]).4,6 Aggressive debridement or radical resection has produced mixed clinical results3,5 (Figure 4). The Canadian Task Force on Osteonecrosis of the Jaw and expert opinion do not support aggressive surgery.4,6,8,10 Hyperbaric oxygen therapy, previously viewed as nonbeneficial,6,8 is now showing effectiveness in clinical studies.3,11
Even though the risk cannot be eliminated, prognosis usually is good for patients with osteonecrosis of the jaw from oral bisphosphonates. The area of bone exposure generally is limited (2 to 10 mm) and of lesser severity, compared with patients with cancer who receive intravenous therapy.2 In a study of osteonecrosis of the jaw induced by oral bisphosphonates, after a four- to nine-month drug holiday, there was complete resolution in 16 of 17 cases: 11 resolved without surgery, three with local debridement, and two with mandibular resection.7
The optimal duration of bisphosphonate treatment for osteoporosis is uncertain. In a randomized controlled trial comparing five versus 10 years of alendronate (Fosamax) therapy, there were statistically significant improvements in bone mineral density and a lower risk of clinical vertebral fractures with 10 years of therapy. However, there were no statistically significant differences in nonvertebral fractures or morphometric vertebral fractures.12 Further investigations are warranted not only because of the diminishing additional benefits, but also because of the increased risks of jaw osteonecrosis and other serious adverse effects from a longer duration of bisphosphonate exposure.3
