
Am Fam Physician. 2012;85(12):1150-1156
A more recent article on hearing loss in adults is available.
Author disclosure: No relevant financial affiliations to disclose.
Hearing loss affects approximately one-third of adults 61 to 70 years of age and more than 80 percent of those older than 85 years. Men usually experience greater hearing loss and have earlier onset compared with women. The most common type is age-related hearing loss; however, many conditions can interfere with the conduction of sound vibrations to the inner ear and their conversion to electrical impulses for conduction to the brain. Screening for hearing loss is recommended in adults older than 50 to 60 years. Office screening tests include the whispered voice test and audioscopy. Older patients who admit to having difficulty hearing may be referred directly for audiometry. The history can identify risk factors for hearing loss, especially noise exposure and use of ototoxic medications. Examination of the auditory canal and tympanic membrane can identify causes of conductive hearing loss. Audiometric testing is required to confirm hearing loss. Adults presenting with idiopathic sudden sensorineural hearing loss should be referred for urgent assessment. Management of hearing loss is based on addressing underlying causes, especially obstructions (including cerumen) and ototoxic medications. Residual hearing should be optimized by use of hearing aids, assistive listening devices, and rehabilitation programs. Surgical implants are indicated for selected patients. Major barriers to improved hearing in older adults include lack of recognition of hearing loss; perception that hearing loss is a normal part of aging or is not amenable to treatment; and patient nonadherence with hearing aids because of stigma, cost, inconvenience, disappointing initial results, or other factors.
At least 28 million U.S. adults have hearing loss.1 After hypertension and arthritis, it is the most common chronic health problem in older persons.2 The impact of hearing loss on society will increase as baby boomers age, because the age-specific prevalence of hearing loss and the number of older persons are increasing.3
Normal conversations use frequencies of 500 to 3,000 Hz at 45 to 60 dB. After 60 years of age, hearing typically declines by about 1 dB annually. Men usually experience greater hearing loss and earlier onset compared with women.4 Hearing loss of 25 dB or more affects about 37 percent of adults 61 to 70 years of age, 60 percent of adults 71 to 80 years of age, and more than 80 percent of adults older than 85 years.5,6 No evidence supports a threshold age for the onset of hearing loss.7
Hearing loss impacts communication and functional ability, and is strongly associated with decreased quality of life, cognitive decline, and depression.3,8 Despite its prevalence and morbidity, hearing loss is underrecognized and undertreated.3 It may be underrecognized because it is a slowly developing problem or because of the belief that hearing loss is a normal part of aging. Undertreatment may result from poor appreciation of options for hearing enhancement, or patient resistance or inability to use hearing aids and assistive listening devices. Cost and social stigma are major factors in the diagnosis and management of hearing loss.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The American Academy of Family Physicians recommends screening persons older than 60 years for hearing loss during periodic health examinations. | C | 13 |
| Older patients who report hearing loss can be referred directly for audiometry. | C | 16 |
| Magnetic resonance imaging with gadolinium is recommended for patients presenting with idiopathic sudden sensorineural hearing loss to identify those with serious underlying pathologic conditions. | C | 22 |
| Appropriate counseling should be provided to patients with hearing loss, because patient perceptions and expectations are the most important factors in the acquisition and use of hearing aids. | C | 20, 30, 31 |
| Referral for assessment for assistive listening devices should be considered in patients with hearing loss who are unable to use hearing aids. | C | 33 |
Etiology and Pathophysiology
Hearing loss is classified as conductive or sensorineural (Table 1). Conductive hearing loss is usually caused by problems in the external or middle ear that interfere with transmitting sound and its conversion to mechanical vibrations. Sensorineural hearing loss involves problems converting mechanical vibrations to electrical potential in the cochlea and/or in auditory nerve transmission to the brain. It is usually caused by permanent damage in the organ of Corti.3 More than 90 percent of older persons with hearing loss have age-related sensorineural hearing loss, which is a gradual, symmetric loss of hearing (predominantly of high frequencies) that is worse in noisy environments.3 Older persons may have both conductive and sensorineural hearing loss, and may also have cognitive difficulties in sound interpretation.
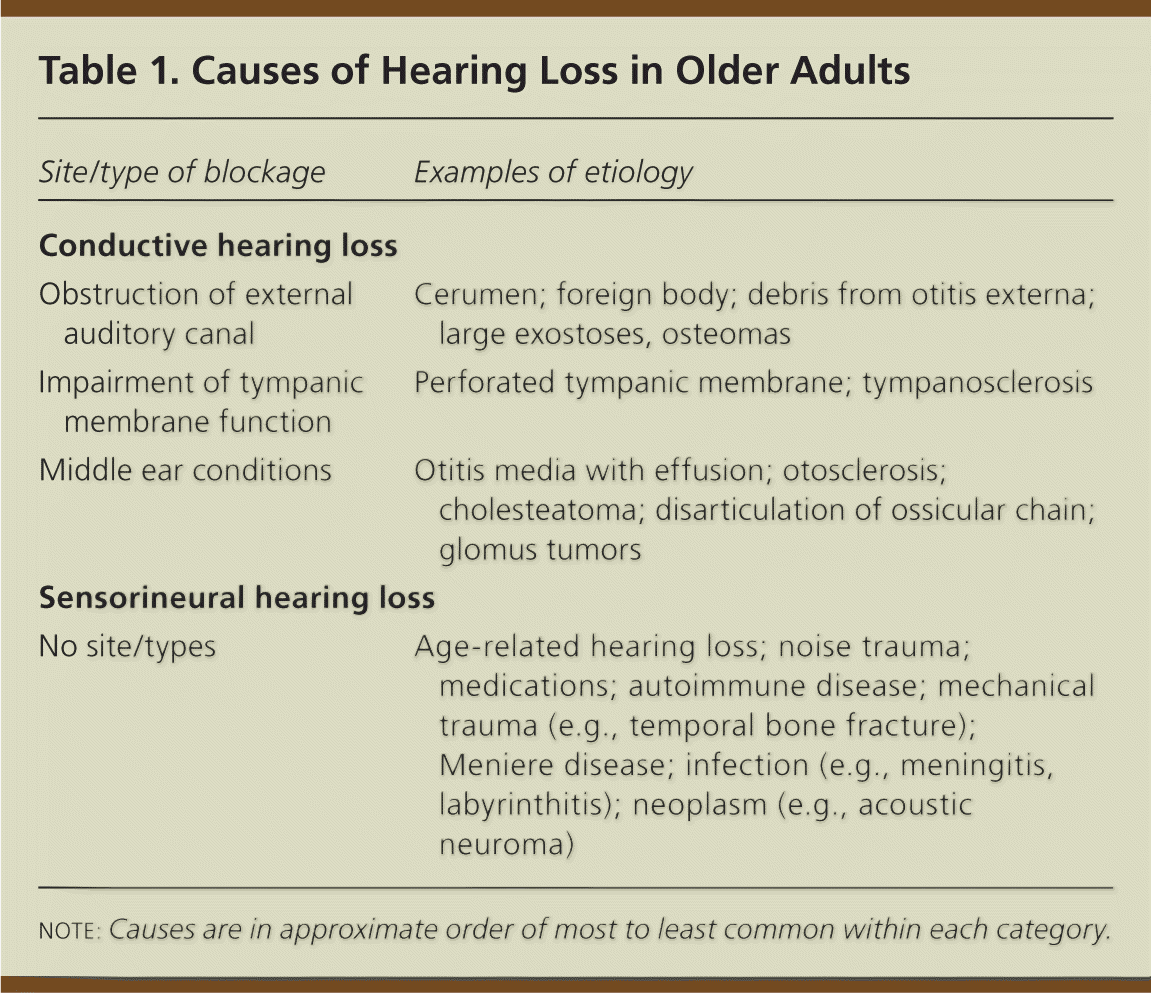
| Site/type of blockage | Examples of etiology |
|---|---|
| Conductive hearing loss | |
| Obstruction of external auditory canal | Cerumen; foreign body; debris from otitis externa; large exostoses, osteomas |
| Impairment of tympanic membrane function | Perforated tympanic membrane; tympanosclerosis |
| Middle ear conditions | Otitis media with effusion; otosclerosis; cholesteatoma; disarticulation of ossicular chain; glomus tumors |
| Sensorineural hearing loss | |
| No site/types | Age-related hearing loss; noise trauma; medications; autoimmune disease; mechanical trauma (e.g., temporal bone fracture); Meniere disease; infection (e.g., meningitis, labyrinthitis); neoplasm (e.g., acoustic neuroma) |
About one-half of the susceptibility to age-related hearing loss may be genetically determined.5 Several genes may be involved.5,9 Noise exposure contributes to the onset, but not to the progression, of age-related hearing loss.7 Regular exposure to 85 dB or more increases the risk of hearing loss by mechanical and metabolic damage to cochlear hair cells. Other implicated factors may be synergistic (Table 2).4,5,9,10
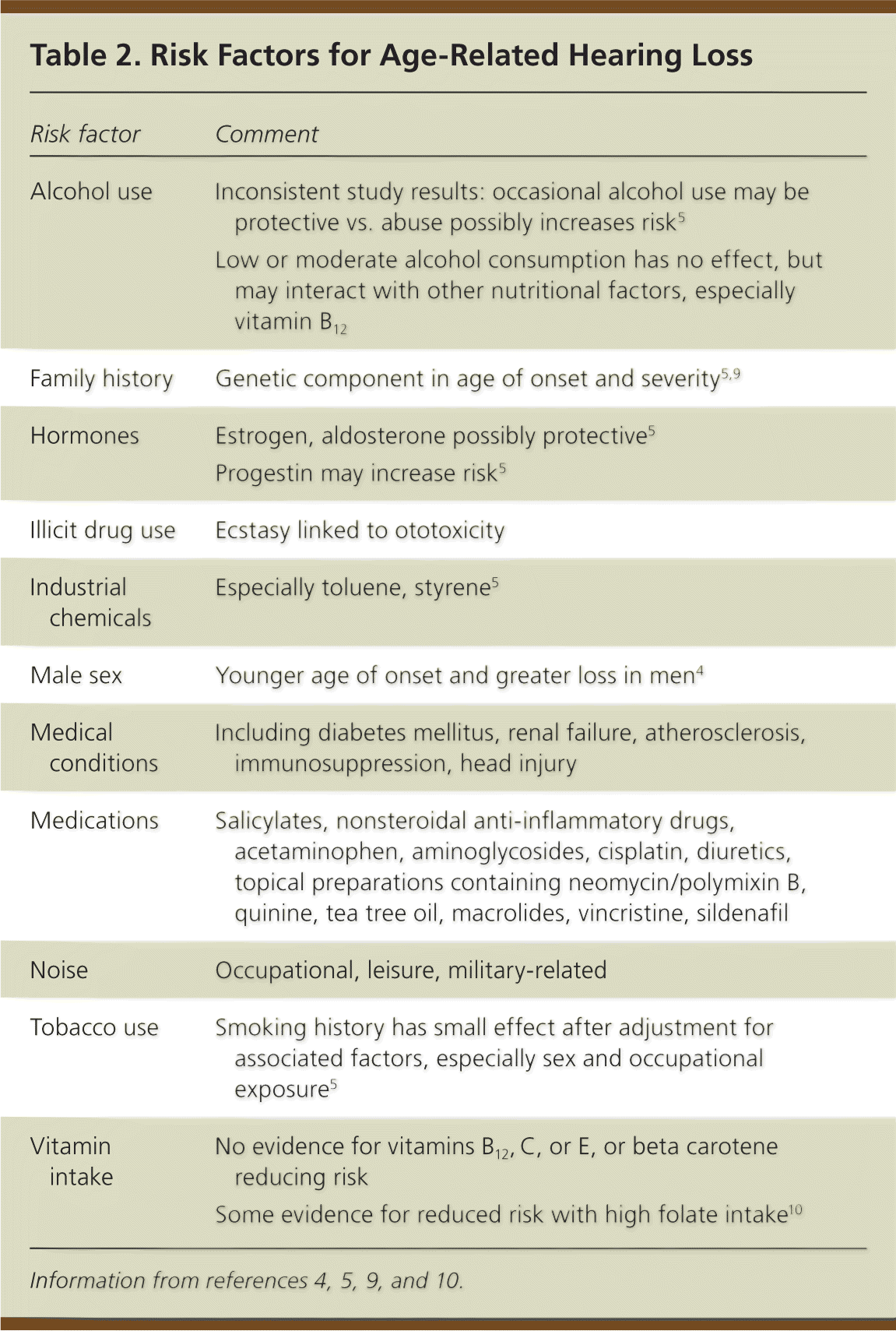
| Risk factor | Comment |
|---|---|
| Alcohol use | Inconsistent study results: occasional alcohol use may be protective vs. abuse possibly increases risk5 |
| Low or moderate alcohol consumption has no effect, but may interact with other nutritional factors, especially vitamin B12 | |
| Family history | Genetic component in age of onset and severity5,9 |
| Hormones | Estrogen, aldosterone possibly protective5 |
| Progestin may increase risk5 | |
| Illicit drug use | Ecstasy linked to ototoxicity |
| Industrial chemicals | Especially toluene, styrene5 |
| Male sex | Younger age of onset and greater loss in men4 |
| Medical conditions | Including diabetes mellitus, renal failure, atherosclerosis, immunosuppression, head injury |
| Medications | Salicylates, nonsteroidal anti-inflammatory drugs, acetaminophen, aminoglycosides, cisplatin, diuretics, topical preparations containing neomycin/polymixin B, quinine, tea tree oil, macrolides, vincristine, sildenafil |
| Noise | Occupational, leisure, military-related |
| Tobacco use | Smoking history has small effect after adjustment for associated factors, especially sex and occupational exposure5 |
| Vitamin intake | No evidence for vitamins B12, C, or E, or beta carotene reducing risk |
| Some evidence for reduced risk with high folate intake10 |
Persons may be unaware of mild to moderate hearing loss because of its insidious onset and progression, or because it is not apparent in quiet environments. Only about 20 percent of persons 65 years or older with moderate to profound hearing loss perceive themselves as hearing impaired.11 Many do not perceive hearing loss as appropriate for medical intervention.
Screening
Formal audiometric testing in a sound-protected environment is the diagnostic standard.12 However, expense and time make this impractical for screening. Good-quality evidence supports common screening tests to identify persons with hearing loss. Nevertheless, the benefits of population screening are difficult to demonstrate because significant numbers of persons do not follow up for audiometric diagnosis. The benefits are further decreased because many persons with confirmed hearing loss do not obtain or use hearing aids.3,11,12
Leading expert organizations recommend asking older patients or caretakers about hearing difficulties. Recommendations differ about further testing if a problem is identified (Table 3).11,13–15 The U.S. Preventive Services Task Force is currently updating its 1996 recommendations.11 The Institute for Clinical Systems Improvement indicates that screening for age-related hearing loss is effective and should be provided whenever possible.15 The American Academy of Family Physicians recommends screening persons older than 60 years during periodic health examinations.13

| Organization | Key issues |
|---|---|
| American Academy of Family Physicians13 | Question older adults about hearing loss, and counsel regarding the availability of treatment when appropriate |
| American Speech-Language-Hearing Association14 | Audiometric testing (25 dB at 1,000, 2,000, and 4,000 Hz) on request or if risk factors are present, and every three years after 50 years of age |
| Institute for Clinical Systems Improvement15 | Ask patients if they have hearing loss, and refer positive responders for formal audiometric testing; screen those who do not perceive any loss using the whispered voice test or audioscopy |
| Screening should be followed by counseling on hearing aids/services, and referrals as appropriate | |
| Screening every two to 10 years is reasonable | |
| U.S. Preventive Services Task Force11 | Good-quality evidence for common tests to identify risk |
| More research needed to clarify net benefits | |
| Updated draft recommendations available for public comment (http://www.uspreventiveservicestaskforce.org/uspstf11/adulthearing/adulthearart.htm) |
Each screening test has advantages and limitations (Table 4).11–13,15–17 A systematic review concluded that adults who report hearing loss (spontaneously or on questioning) should be referred directly for audiometry, and those who deny hearing problems should be screened with the whispered voice test or audioscopy.16 Inspection of the auditory canal and tympanic membrane, removal of any obstruction, and repeat questioning are prudent before screening or referral. The screening version of the Hearing Handicap Inventory for the Elderly provides information on social and emotional problems (Table 5).18 It can be used alone or to complement other tests.12 Studies indicate that telephone or Internet-based screening may also be effective.17
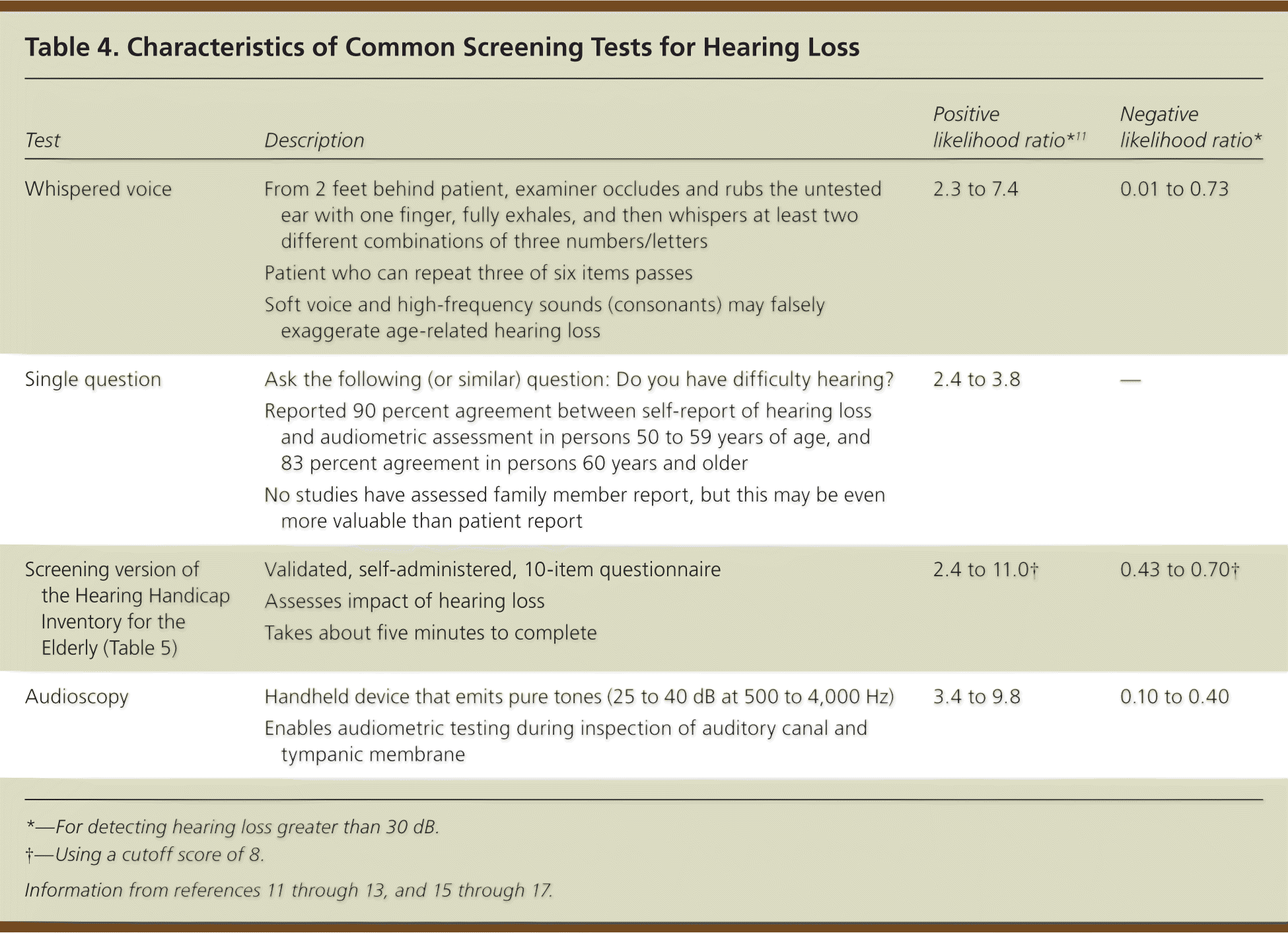
| Test | Description | Positive likelihood ratio*11 | Negative likelihood ratio* |
|---|---|---|---|
| Whispered voice | From 2 feet behind patient, examiner occludes and rubs the untested ear with one finger, fully exhales, and then whispers at least two different combinations of three numbers/letters | 2.3 to 7.4 | 0.01 to 0.73 |
| Patient who can repeat three of six items passes | |||
| Soft voice and high-frequency sounds (consonants) may falsely exaggerate age-related hearing loss | |||
| Single question | Ask the following (or similar) question: Do you have difficulty hearing? | 2.4 to 3.8 | – |
| Reported 90 percent agreement between self-report of hearing loss and audiometric assessment in persons 50 to 59 years of age, and 83 percent agreement in persons 60 years and older | |||
| No studies have assessed family member report, but this may be even more valuable than patient report | |||
| Screening version of the Hearing Handicap Inventory for the Elderly (Table 5) | Validated, self-administered, 10-item questionnaire | 2.4 to 11.0† | 0.43 to 0.70† |
| Assesses impact of hearing loss | |||
| Takes about five minutes to complete | |||
| Audioscopy | Handheld device that emits pure tones (25 to 40 dB at 500 to 4,000 Hz) | 3.4 to 9.8 | 0.10 to 0.40 |
| Enables audiometric testing during inspection of auditory canal and tympanic membrane |

| 1. Does a hearing problem cause you to feel embarrassed when meeting new people? |
| 2. Does a hearing problem cause you to feel frustrated when talking to members of your family? |
| 3. Do you have difficulty hearing when someone speaks in a whisper? |
| 4. Do you feel handicapped by a hearing problem? |
| 5. Does a hearing problem cause you difficulty when visiting friends, relatives, or neighbors? |
| 6. Does a hearing problem cause you to attend religious services less often than you would like? |
| 7. Does a hearing problem cause you to have arguments with family members? |
| 8. Does a hearing problem cause you difficulty when listening to the television or radio? |
| 9. Do you feel that any difficulty with your hearing limits or hampers your personal or social life? |
| 10. Does a hearing problem cause you difficulty when in a restaurant with relatives or friends? |
Assessment
The history may indicate the etiology of hearing loss (Table 6), and can identify risk factors, especially noise exposure and use of ototoxic medications (Table 24,5,9,10 ). Unilateral hearing loss suggests local pathology, obstruction, or idiopathic sudden sensorineural hearing loss. Gradual onset may suggest age-related hearing loss, otosclerosis, or acoustic neuroma. By comparison, rapid onset may be related to a perforated tympanic membrane, trauma, or idiopathic sudden sensorineural hearing loss. The history should include asking about functional and emotional impacts of hearing loss, especially depression and social isolation.

| Item | Finding and significance |
|---|---|
| Laterality | Unilateral hearing loss suggests local pathology, obstruction, or idiopathic sudden sensorineural hearing loss |
| Onset | Gradual onset suggests age-related hearing loss, otosclerosis, acoustic neuroma |
| Rapid onset suggests perforated tympanic membrane, trauma, idiopathic sudden sensorineural hearing loss | |
| Inciting factors | Temporal relationship to ototoxic medications/substances, infections, trauma |
| Associated features | Exacerbation in noisy environments and greater loss of high frequencies suggest age-related hearing loss |
| Harsh, distorted sound and aural fullness typical of idiopathic sudden sensorineural hearing loss | |
| Other symptoms depending on underlying pathology (e.g., tinnitus, nausea, vertigo in persons with Meniere disease; neurologic findings in persons with tumors) |
Physical examination includes inspection of the auditory canal and tympanic membrane for obstruction. Tympanic membrane mobility testing requires a pneumatic bulb. If indicated by the history, examination of the head and neck, sinuses, oropharynx, and cranial nerves, and evaluation of vestibular, cerebellar, and cognitive function may be helpful; however, no evidence-based recommendations are available.16
The Weber and Rinne tests were designed to distinguish conductive from sensorineural hearing loss by comparing air and bone conduction. A review concluded that these tests should not be used routinely for screening because of accuracy problems.16
Laboratory and imaging tests should be individualized by patient presentation.
Management
Management goals are to address any underlying, contributing, or comorbid conditions and to optimize hearing. Effective intervention can improve social and emotional function, communication, cognitive function, and depression for patients with hearing loss.19 Despite the potential quality-of-life benefits for patients and families, nonadherence with treatment recommendations is common. The patient's perception of the significance of hearing loss, expectations for improvement, and willingness to use assistive listening devices permanently are significant factors in adherence. Disappointing initial results with hearing aids is a commonly cited reason for nonadherence.20
Additional influences include convenience, cost, type of intervention (especially the design of aids and devices), social norms, negative stereotypes associated with hearing loss and/or use of hearing aids, reported experience of others, and physician recommendations.21 Treatment strategies for hearing loss must be patient-centered and individualized.20 A major role for family physicians is identifying and addressing patient barriers to management of hearing loss, encouraging adherence with the optimal interventions, and monitoring for improvements in management as the patient's situation changes and/or new treatments, aids, and devices become available.
IDIOPATHIC SUDDEN SENSORINEURAL HEARING LOSS
Idiopathic sudden sensorineural hearing loss develops in less than 72 hours and is usually unilateral. The sound is described as harsh and distorted with accompanying aural fullness. It affects five to 20 per 100,000 adults 40 to 60 years of age annually.22 Approximately 32 to 70 percent recover spontaneously, but idiopathic sudden sensorineural hearing loss is an emergency requiring prompt referral. Up to 16 percent of patients presenting with idiopathic sudden sensorineural hearing loss are subsequently diagnosed with significant pathology, including autoimmune disease and neurologic conditions. Magnetic resonance imaging with gadolinium is recommended for all patients with potential idiopathic sudden sensorineural hearing loss to identify those with serious underlying pathologic conditions.22
Steroids are the current standard treatment for idiopathic sudden sensorineural hearing loss, but several systematic reviews have failed to support significantly better outcomes with steroid therapy compared with placebo or other treatments.22–25 Studies have also failed to identify predictive factors for spontaneous recovery in idiopathic sudden sensorineural hearing loss.26 Because of the risk of permanent hearing loss and the possibility of symptoms being caused by another serious condition, patients presenting with symptoms suggesting idiopathic sudden sensorineural hearing loss should be referred for urgent specialist assessment.22
CONDUCTIVE HEARING LOSS
Conductive hearing loss often exacerbates age-related hearing loss. The most common cause, cerumen impaction, may exacerbate hearing loss in up to 30 percent of older persons. Techniques for removing cerumen include curetting, nonprescription solutions (hydrogen peroxide–based), warm water irrigation, and prescription cerumenolytics. A comprehensive review including studies of 11 different agents concluded that drops of any type appear to provide better outcomes than no intervention, but no specific drop is more effective than another.27 Although warm water irrigation is commonly used, one study demonstrated hearing improvement in only 34 percent of persons after visually effective irrigation.28
Chronic otitis media with effusion (serous otitis media) is common in older patients, but treatment has been evaluated only in children. Short courses of oral steroids or antibiotics may ameliorate serous otitis media. Patients with persistent serous otitis media should be referred for assessment because of the possibility of nasopharyngeal carcinoma or another lesion obstructing the eustachian tube.3
HEARING AIDS
Hearing aids are available as behind-the-ear, in-the-ear, and in-the-canal models. The choice of aid is predominantly determined by the patient's perception of ease of use and appearance.3 Practical problems include inserting, removing, and cleaning the device; changing batteries; controlling volume; and coping with extraneous noises, especially “whistles.” In-the-ear aids may be the easiest to manage, but patient satisfaction correlates better with the size and shape of the aid than the type.29 Patients are more likely to use in-the-canal or in-the-ear aids than behind-the-ear models.3
Despite many well-documented benefits, only 25 percent of eligible patients acquire hearing aids and up to 30 percent of those do not use their aids.19,30 The biggest motivation for use is patient confidence that communication will improve. The biggest barrier is the perception that hearing loss does not merit correction, regardless of the level of loss.31 Hearing aid use is not correlated with age, education level, functional impairment, or medication use.32 Physicians can play a significant role in promoting the proper use of hearing aids. Counseling should be provided to patients with hearing loss, because patient perceptions and expectations are the most important factors in the acquisition and use of hearing aids.20,30,31
ASSISTIVE LISTENING DEVICES
Referral for assessment for assistive listening devices should be considered in patients with hearing loss who are unable to use hearing aids.33 Assistive listening devices can provide visual or tactile alerts or amplify other devices, such as telephones. Assistive listening devices provide safe, cost-effective alternatives to hearing aids for some older persons, such as those who are bed-bound, who are reliant on others for hearing aid management, or who could swallow or lose aids because of cognitive impairment.33 They may be useful adjuncts to hearing aids. Assistive listening devices are most successful in improving quality of life when individual patient needs are closely matched to specific devices.
SURGICAL IMPLANTS
In selected patients, including those who do not tolerate hearing aids, middle ear implants may provide comparable improvement in sound quality and clarity to hearing aids. Preliminary studies suggest that the implants improve hearing and patient satisfaction mainly by reducing symptoms of ear occlusion and sound feedback.34 Cost is a major limitation of more widespread use of these implants.
In patients with severe sensorineural hearing loss, cochlear implants can improve sound discrimination better than other devices.35,36 A recent small retrospective study showed that even in adults older than 70 years, cochlear implants improved quality of life and decreased tinnitus.37 More widespread use of cochlear implants and other surgical treatments for older patients will depend on evidence that the potential for enhanced function and quality of life can be achieved despite adverse factors, mainly surgical risk and cost.38
REHABILITATION
Communication therapy programs provide training in utilizing nonverbal cues and enhanced communication skills, particularly in noisy environments. Programs can improve communication as well as psychological well-being.39 Providing training that enhances communication skills to spouses of persons with hearing loss also reduces stress and improves communication and quality of life for both partners.40
Prevention
Avoiding risk factors and noise is key to preventing the onset of age-related hearing loss. Emerging evidence suggests that folic acid (800 mcg daily) and high intake of omega-3 fatty acids may slow hearing decline.10,41 Conversely, elevated homocysteine levels may predispose to more rapid or more severe hearing loss.42 Additional research is needed on potential strategies to prevent the onset and slow the progression of age-related hearing loss.
Data Sources: A PubMed search was completed using the key terms hearing loss, older adults, and hearing impairment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. The Agency for Healthcare Research and Quality evidence reports, the Cochrane database, the National Guideline Clearinghouse database, Clinical Evidence, and Essential Evidence Plus were also searched. Searches were conducted initially in December 2010. Additional searches were conducted in January 2011, and September through October 2011. Secondary searches were based on the bibliographies of the articles and resources identified by the primary searches.
editor's note: Anne D. Walling, MB, ChB, FFPHM is an associate medical editor for American Family Physician.
