Editor's Note: In violation of our conflict of interest policy, Dr. Ament failed to disclose financial relationships with two pharmaceutical companies that existed at the time of submission and publication of this article. His disclosure statement has been corrected below.
Am Fam Physician. 2012;86(1):66-70
Related letter: Unsupported Risks of Proton Pump Inhibitor Use
Patient information: See related handout on side effects of proton pump inhibitors, written by the authors of this article.
[ corrected] Author disclosure: Dr. Ament reports that he serves on the speakers’ bureaus of Pfizer and Merck, and that he is on the speakers’ bureau of Sanofi Aventis regarding enoxaparin (Lovenox).
Proton pump inhibitors effectively treat gastroesophageal reflux disease, erosive esophagitis, duodenal ulcers, and pathologic hypersecretory conditions. Proton pump inhibitors cause few adverse effects with short-term use; however, long-term use has been scrutinized for appropriateness, drug-drug interactions, and the potential for adverse effects (e.g., hip fractures, cardiac events, iron deficiency, Clostridium difficile infection, pneumonia). Adults 65 years and older are more vulnerable to these adverse effects because of the higher prevalence of chronic diseases in this population. Proton pump inhibitors administered for stress ulcer prophylaxis should be discontinued after the patient is discharged from the intensive care unit unless other indications exist.
In 1990, the U.S. Food and Drug Administration (FDA) approved omeprazole (Prilosec), the first proton pump inhibitor (PPI), for the short-term treatment of gastroesophageal reflux disease, active duodenal ulcer, severe erosive esophagitis, and pathologic hypersecretory conditions.1 The mechanism of action for PPIs involves blocking the terminal pathway that stimulates gastric acid release.2,3 The PPI class now includes at least seven other drugs, including parenteral and over-the-counter formulations (Table 1).
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| PPI use is associated with an increased risk of fractures of the hip, wrist, and spine. | B | 12–14 | — |
| PPI therapy in combination with clopidogrel (Plavix) use may increase the risk of cardiac events. | B | 18–25 | Limited clinical data supporting increased cardiovascular risk with this combination |
| Product labeling change recommends avoiding combination therapy with omeprazole (Prilosec) and clopidogrel | |||
| PPI use increases the risk of Clostridium difficile infection. | C | 6–8 | Consistent findings from prospective and retrospective studies |
| PPI use increases the risk of community-acquired pneumonia. | B | 9–11 | Consistent findings from case-control studies |
| Discontinuation of PPI therapy is associated with symptoms of gastroesophageal reflux disease from gastric acid rebound. | B | 15, 16 | Randomized double-blind studies demonstrate symptomatic gastric acid rebound lasting several weeks after discontinuation of PPI therapy |
| PPI use is effective in preventing stress ulcers in patients in the intensive care unit. | B | 30 | Practice guideline recommends stress ulcer prophylaxis in select patients admitted to the intensive care unit |
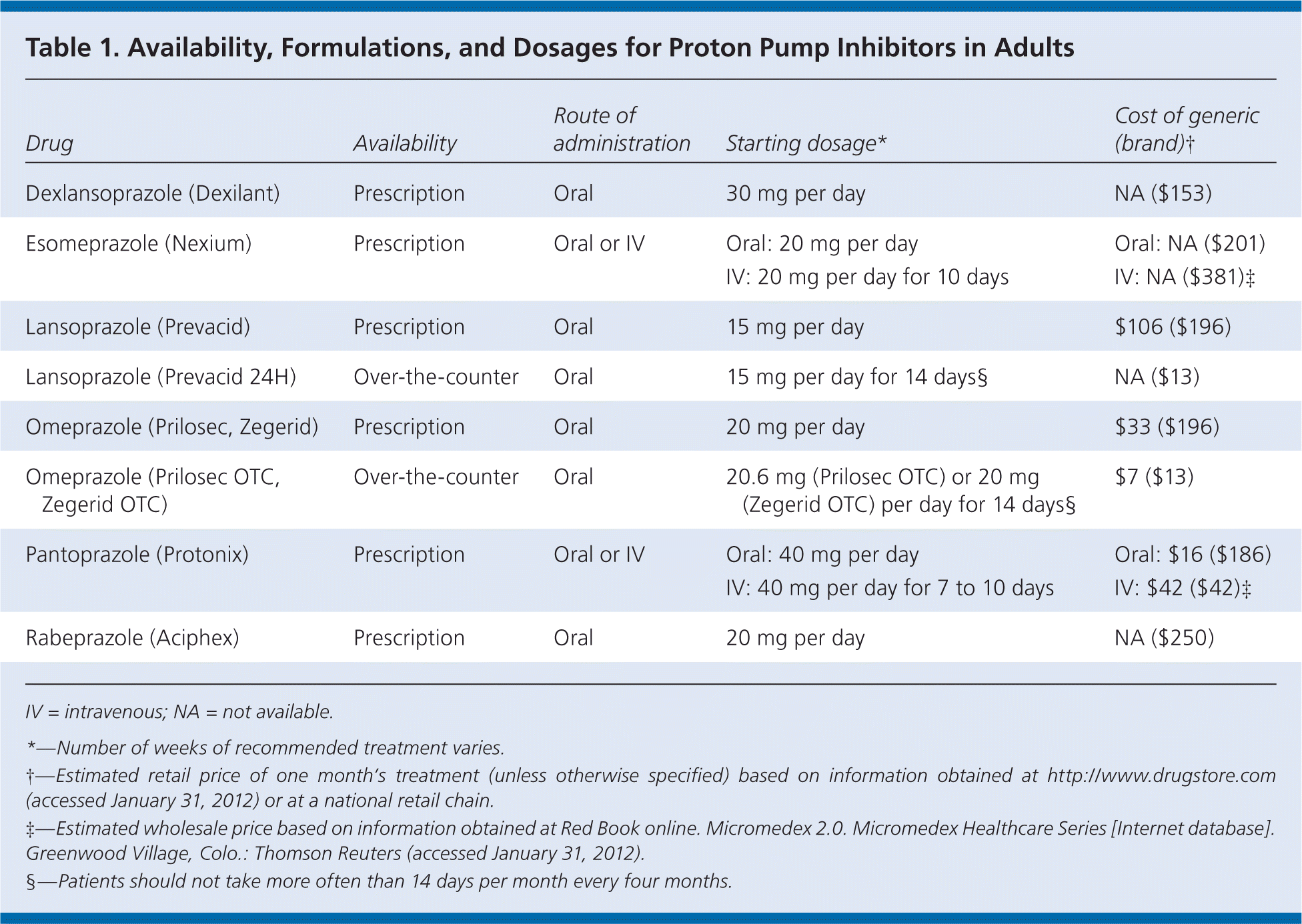
| Drug | Availability | Route of administration | Starting dosage* | Cost of generic (brand)† |
|---|---|---|---|---|
| Dexlansoprazole (Dexilant) | Prescription | Oral | 30 mg per day | NA ($153) |
| Esomeprazole (Nexium) | Prescription | Oral or IV | Oral: 20 mg per day | Oral: NA ($201) |
| IV: 20 mg per day for 10 days | IV: NA ($381)‡ | |||
| Lansoprazole (Prevacid) | Prescription | Oral | 15 mg per day | $106 ($196) |
| Lansoprazole (Prevacid 24H) | Over-the-counter | Oral | 15 mg per day for 14 days§ | NA ($13) |
| Omeprazole (Prilosec, Zegerid) | Prescription | Oral | 20 mg per day | $33 ($196) |
| Omeprazole (Prilosec OTC, Zegerid OTC) | Over-the-counter | Oral | 20.6 mg (Prilosec OTC) or 20 mg (Zegerid OTC) per day for 14 days§ | $7 ($13) |
| Pantoprazole (Protonix) | Prescription | Oral or IV | Oral: 40 mg per day | Oral: $16 ($186) |
| IV: 40 mg per day for 7 to 10 days | IV: $42 ($42)‡ | |||
| Rabeprazole (Aciphex) | Prescription | Oral | 20 mg per day | NA ($250) |
Currently, the PPI class is the third highest selling drug category in the United States, accounting for more than 113 million prescriptions annually with sales exceeding $14 billion.4 Patients often continue therapy for extended durations without an end point. Studies have found that up to 70 percent of PPI use is for unapproved indications.4
Adverse Effects
The overuse and misuse of PPIs are concerning. PPIs cause few adverse effects with short-term use; however, long-term PPI use has been associated with an increased risk of all-cause mortality in two cohorts of institutionalized older persons,5 in addition to being linked to a number of adverse effects (Table 26–23 ). Adults 65 years and older are at higher risk of these adverse effects because of the higher prevalence of chronic diseases in this population.
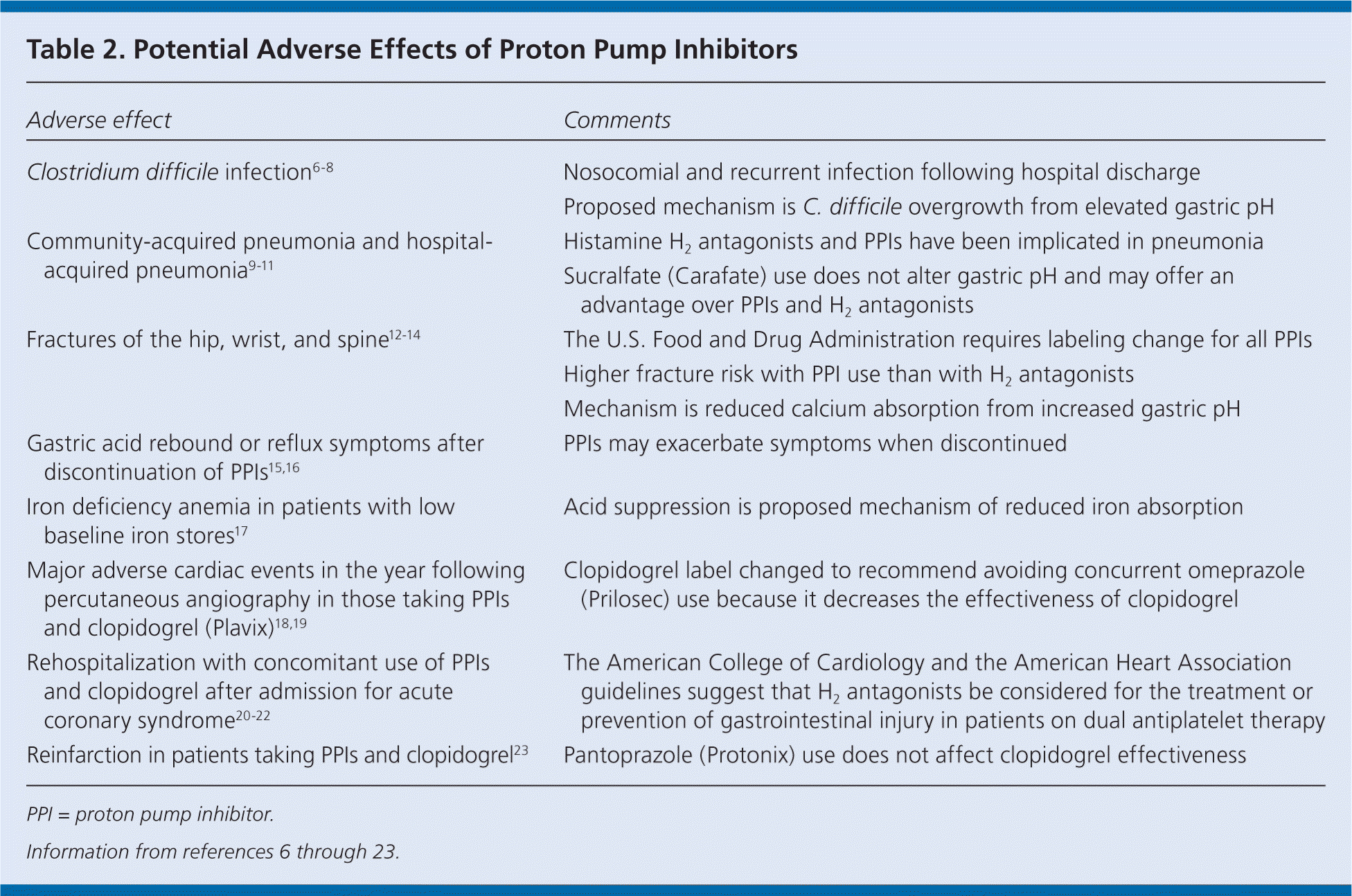
| Adverse effect | Comments |
|---|---|
| Clostridium difficile infection6–8 | Nosocomial and recurrent infection following hospital discharge |
| Proposed mechanism is C. difficile overgrowth from elevated gastric pH | |
| Community-acquired pneumonia and hospital-acquired pneumonia9–11 | Histamine H2 antagonists and PPIs have been implicated in pneumonia |
| Sucralfate (Carafate) use does not alter gastric pH and may offer an advantage over PPIs and H2 antagonists | |
| Fractures of the hip, wrist, and spine12–14 | The U.S. Food and Drug Administration requires labeling change for all PPIs |
| Higher fracture risk with PPI use than with H2 antagonists | |
| Mechanism is reduced calcium absorption from increased gastric pH | |
| Gastric acid rebound or reflux symptoms after discontinuation of PPIs15,16 | PPIs may exacerbate symptoms when discontinued |
| Iron deficiency anemia in patients with low baseline iron stores17 | Acid suppression is proposed mechanism of reduced iron absorption |
| Major adverse cardiac events in the year following percutaneous angiography in those taking PPIs and clopidogrel (Plavix)18,19 | Clopidogrel label changed to recommend avoiding concurrent omeprazole (Prilosec) use because it decreases the effectiveness of clopidogrel |
| Rehospitalization with concomitant use of PPIs and clopidogrel after admission for acute coronary syndrome20–22 | The American College of Cardiology and the American Heart Association guidelines suggest that H2 antagonists be considered for the treatment or prevention of gastrointestinal injury in patients on dual antiplatelet therapy |
| Reinfarction in patients taking PPIs and clopidogrel23 | Pantoprazole (Protonix) use does not affect clopidogrel effectiveness |
HIP FRACTURE
A retrospective study in the United Kingdom found a substantially higher incidence of hip fractures in patients older than 50 years who used PPIs for more than one year, with higher fracture risk associated with longer treatment duration.12 A similar study found an increased fracture risk in patients using long-term PPIs who had at least one other risk factor, including diabetes mellitus, chronic renal diseases, and glucocorticoid use.13 In 2010, the FDA revised the labeling of all PPIs to include the increased risk of fractures of the hip, wrist, and spine.14
CARDIAC EVENTS
Risk factors for gastrointestinal bleeding in patients taking clopidogrel (Plavix) include previous gastrointestinal bleeding; advanced age; concomitant use of warfarin (Coumadin), glucocorticoids, or nonsteroidal anti-inflammatory drugs; and Helicobacter pylori infection. In 2007, the American College of Cardiology and the American Heart Association recommended the use of gastroprotective agents in patients with unstable angina or non–ST segment elevation myocardial infarction who were taking aspirin and clopidogrel, and who had a history of gastrointestinal ulceration.20 Since that time, studies have suggested an increased risk of reinfarction in patients taking clopidogrel and a PPI other than pantoprazole (Protonix).23 There also may be an increased risk of rehospitalization with concomitant use of a PPI and clopidogrel following percutaneous angiography and treatment for acute coronary syndrome.18,21 Therefore, the American College of Cardiology and the American Heart Association suggest that physicians prescribe drugs other than PPIs, such as histamine H2 antagonists (e.g., ranitidine [Zantac]), for patients taking aspirin and clopidogrel who require gastroprotection.22
Studies show that PPIs block the effects of clopidogrel by inhibiting cytochrome P450 2C19 isozyme (CYP2C19), which converts clopidogrel to its active form. Although data strongly suggest an interaction between omeprazole and clopidogrel, the clinical significance of this interaction is unknown.22 Pantoprazole may be preferred over other PPIs in the setting of acute coronary syndrome or coronary stenting because it has a lower potential to inhibit CYP2C19.24
In patients with concomitant atherosclerosis and a high risk of gastrointestinal bleeding, physicians should weigh the potential cardiovascular risks of PPIs against less effective alternatives, such as H2 antagonists.22,25 The product information for clopidogrel has been changed to recommend avoiding concurrent use with omeprazole because it decreases the antiplatelet effectiveness of clopidogrel.19
IRON DEFICIENCY
An increase in gastric pH is a normal physiologic change in the gastrointestinal tract of older persons and is exacerbated by long-term PPI use. It is unlikely that patients with normal iron stores will develop iron deficiency anemia solely from PPI use. However, patients with low baseline iron stores may be more susceptible to further iron depletion with concurrent PPI therapy.17
ENTERIC INFECTIONS
Studies indicate an increased risk of diarrhea from Clostridium difficile and of other enteric infections in hospitalized patients taking PPIs and antibiotics.6,7 There is also an increased risk of recurrent C. difficile infection after hospital discharge in those taking PPIs.8 Acid suppression has been associated with an increased risk of Campylobacter enteritis. An increase in acute viral gastroenteritis in children was reported in those treated with PPIs for gastroesophageal reflux disease.26 The proposed mechanism of action is a less acidic gastric environment, leading to increased bacterial colonization of the upper gastrointestinal tract.27
PNEUMONIA
Acid suppression allows ingested pathogens to colonize the gastrointestinal tract and relocate to the respiratory tract.28 One study of older patients hospitalized for pneumonia found that initiating a PPI or H2 antagonist at discharge significantly increased the likelihood of recurrent community-acquired pneumonia.9 Another study found a statistically significant increase in the risk of hospital-acquired pneumonia with PPI use.10 Physicians should weigh the risks versus benefits before initiating a PPI in patients being treated for pneumonia.11
GASTRIC ACID REBOUND
Several studies have demonstrated that asymptomatic patients who are administered short-course PPI therapy develop rebound gastrointestinal symptoms after discontinuation.15,16 Patients reported clinically significant dyspeptic symptoms after discontinuation of a two-month course of esomeprazole (Nexium) compared with placebo (44 versus 15 percent).15 A similar study of pantoprazole in asymptomatic, H. pylori–negative patients showed persistent rebound gastrointestinal symptoms for two weeks following discontinuation.16
Stress Ulcer Prophylaxis
Many patients admitted to the intensive care unit (ICU) have risk factors for stress ulcers, with active bleeding producing a higher rate of mortality.29 Significant risk factors associated with gastrointestinal bleeding include coagulopathy and mechanical ventilation exceeding 48 hours’ duration.30 Based on a review of 58 studies, stress ulcer prophylaxis is recommended by the Eastern Association for the Surgery of Trauma for patients with any of the following: mechanical ventilation longer than 48 hours, coagulopathy (e.g., platelet count less than 50 × 103 per μL [50 × 109 per L], international normalized ratio greater than 1.5, partial thromboplastin time greater than two times the normal control), traumatic brain injury, and major burn injury (more than 30 percent of body surface).30
Effective options for stress ulcer prophylaxis include PPIs, H2 antagonists, antacids, and sucralfate (Carafate). No medication has been shown to be superior to another. Although the optimal duration of prophylaxis is not known, most experts suggest continuing therapy while the patient is in the ICU, when bleeding risk is highest. However, many patients continue to receive prophylaxis inappropriately when they are transferred to general medical units and continue therapy after discharge without clear medical indications.31 To minimize adverse outcomes, physicians should discontinue PPIs in patients when they are discharged from the ICU if there are no other indications for therapy.
Data Sources: A literature search was performed in PubMed including clinical reviews, randomized controlled trials, and meta-analyses. The following search terms were used: proton pump inhibitor, adverse effects, side effects, indications, and cardiovascular. Additional sources were obtained from the Eastern Association for the Surgery of Trauma Guidelines. Search dates: December 2010 to February 2011.