
Am Fam Physician. 2012;86(2):192-195
Author disclosure: No relevant financial affiliations to disclose.
Clinical Question
What is the best medication for the treatment of motion sickness?
Evidence-Based Answer
Scopolamine should be used to reduce nausea associated with motion sickness, but it does not reduce vomiting. (Strength of Recommendation [SOR]: A, based on multiple randomized controlled trials [RCTs].) First-generation antihistamines (dimenhydrinate and chlorpheniramine) can also be used to reduce nausea associated with motion sickness. (SOR: B, based on multiple RCTs.) Scopolamine is more effective than meclizine (Antivert) and as effective as dimenhydrinate. Ondansetron (Zofran) and the second-generation antihistamines cetirizine (Zyrtec) and fexofenadine (Allegra) do not reduce symptoms of motion sickness and should not be used. (SOR: B, based on small RCTs.) Ginger can be used to reduce symptoms of motion sickness. (SOR: B, based on RCTs with conflicting results.)
Evidence Summary
SCOPOLAMINE
A Cochrane review of 14 RCTs with a total of 1,025 participants who had sea- or lab-induced motion sickness compared scopolamine with placebo and various other agents.1 Scopolamine reduced nausea more than placebo (relative risk reduction = 0.47; 95% confidence interval, 0.31 to 0.71) but did not reduce vomiting. Patients receiving scopolamine were more likely to have dry mouth (a 22 to 50 percent increase). Three of the RCTs compared scopolamine with antihistamines. Two studies found scopolamine to be superior to meclizine, and one found it to be equivalent to dimenhydrinate.1
FIRST-GENERATION ANTIHISTAMINES
Numerous histamine H1 receptor antagonists are available over the counter and by prescription, including dimenhydrinate, chlorpheniramine, diphenhydramine (Benadryl), and meclizine. One small RCT (n = 16) found that dimenhydrinate reduced nausea scores more than placebo, and another found that high-dose (12-mg) chlorpheniramine reduced the risk of severe malaise more than placebo2,3 (Table 11–10 ). A higher incidence of dry mouth was found with dimenhydrinate, and more sedation was reported with chlorpheniramine.
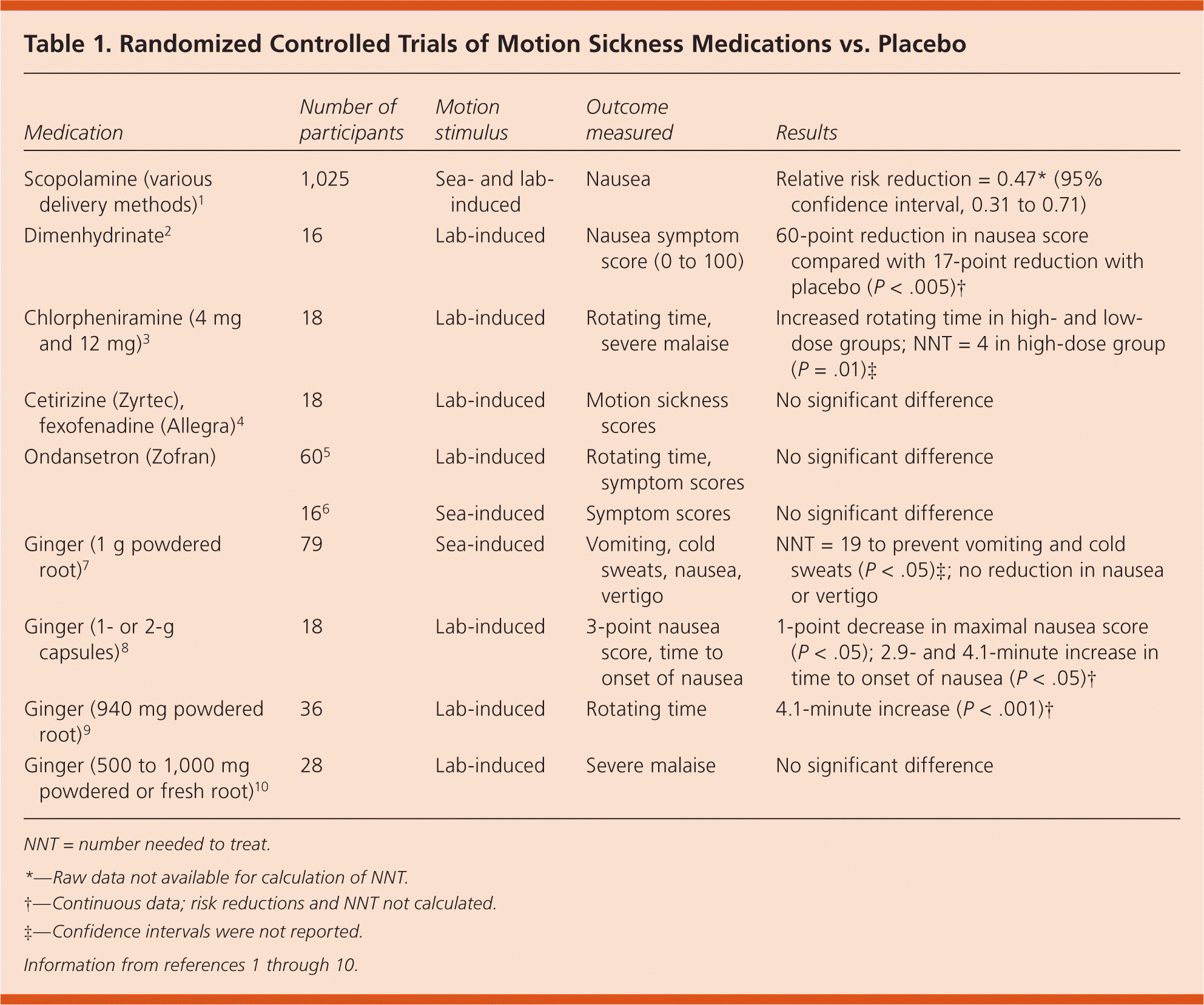
| Medication | Number of participants | Motion stimulus | Outcome measured | Results |
|---|---|---|---|---|
| Scopolamine (various delivery methods)1 | 1,025 | Sea- and lab-induced | Nausea | Relative risk reduction = 0.47* (95% confidence interval, 0.31 to 0.71) |
| Dimenhydrinate2 | 16 | Lab-induced | Nausea symptom score (0 to 100) | 60-point reduction in nausea score compared with 17-point reduction with placebo (P < .005)† |
| Chlorpheniramine (4 mg and 12 mg)3 | 18 | Lab-induced | Rotating time, severe malaise | Increased rotating time in high- and low-dose groups; NNT = 4 in high-dose group (P = .01)‡ |
| Cetirizine (Zyrtec), fexofenadine (Allegra)4 | 18 | Lab-induced | Motion sickness scores | No significant difference |
| Ondansetron (Zofran) | 605 | Lab-induced | Rotating time, symptom scores | No significant difference |
| 166 | Sea-induced | Symptom scores | No significant difference | |
| Ginger (1 g powdered root)7 | 79 | Sea-induced | Vomiting, cold sweats, nausea, vertigo | NNT = 19 to prevent vomiting and cold sweats (P < .05)‡; no reduction in nausea or vertigo |
| Ginger (1- or 2-g capsules)8 | 18 | Lab-induced | 3-point nausea score, time to onset of nausea | 1-point decrease in maximal nausea score (P < .05); 2.9- and 4.1-minute increase in time to onset of nausea (P < .05)† |
| Ginger (940 mg powdered root)9 | 36 | Lab-induced | Rotating time | 4.1-minute increase (P < .001)† |
| Ginger (500 to 1,000 mg powdered or fresh root)10 | 28 | Lab-induced | Severe malaise | No significant difference |
SECOND-GENERATION ANTIHISTAMINES
One RCT with 18 healthy participants evaluated the second-generation, nonsedating antihistamines cetirizine and fexofenadine in lab-induced motion sickness, and found no statistically significant difference in motion sickness scores compared with placebo.4
ONDANSETRON
GINGER
Two higher-quality, placebo-controlled RCTs found that ginger reduced vomiting (but not nausea) in the larger trial, and delayed the onset and reduced the intensity of nausea in the smaller trial.7,8 Two older RCTs comparing ginger with placebo for lab-induced motion sickness produced conflicting results; one found that ginger delayed the onset of nausea, whereas the other found no difference in severe malaise.9,10
Recommendations from Others
Based on a summary of the evidence and expert opinion, UpToDate recommends the use of sedating antihistamines, scopolamine, or ginger for the treatment of motion sickness.11
