
Am Fam Physician. 2012;86(4):370-374
Guideline source: Centers for Disease Control and Prevention
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Not reported
Published source:Morbidity and Mortality Weekly Report, December 9, 2011; published correction appears in Morbidity and Mortality Weekly Report, February 3, 2012
Tuberculosis is a contagious and potentially fatal disease transmitted by Mycobacterium tuberculosis. Approximately 5 to 10 percent of persons infected with the bacterium develop tuberculosis, usually after a latency period of six to 18 months or more. The traditional preventive therapy for persons with latent M. tuberculosis infection involves a daily regimen of isoniazid for nine months; however, completion rates are low (60 percent or less) because of the long duration of therapy. After reviewing three randomized controlled trials, the Centers for Disease Control and Prevention (CDC) has issued recommendations for the treatment of latent M. tuberculosis infection. The new regimen consists of isoniazid and rifapentine (Priftin) administered once per week for 12 weeks as directly observed therapy (Table 1). The reviewed studies demonstrated this regimen to be as effective as nine months of isoniazid monotherapy for preventing tuberculosis.
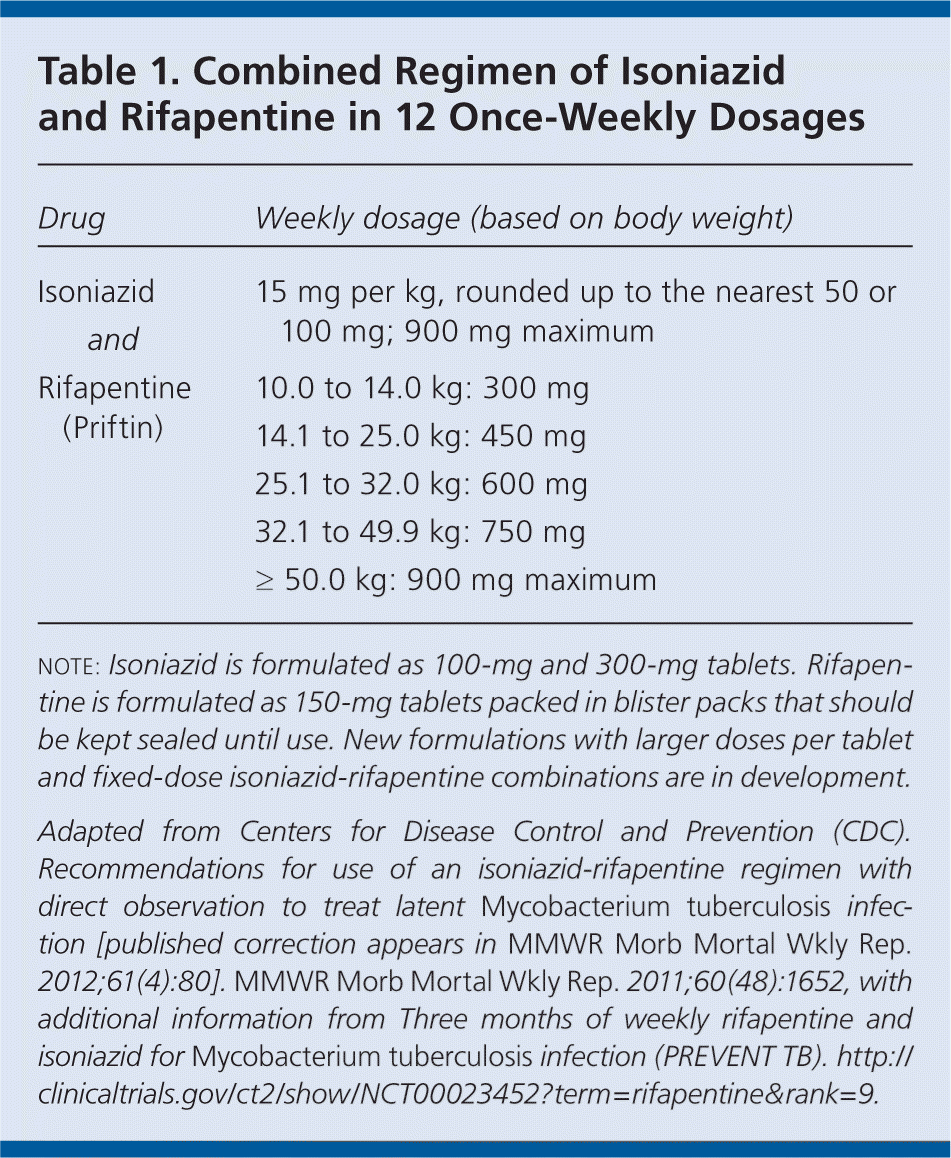
| Drug | Weekly dosage (based on body weight) | |
|---|---|---|
| Isoniazid | 15 mg per kg, rounded up to the nearest 50 or 100 mg; 900 mg maximum | |
| and | ||
| Rifapentine (Priftin) | 10.0 to 14.0 kg: 300 mg | |
| 14.1 to 25.0 kg: 450 mg | ||
| 25.1 to 32.0 kg: 600 mg | ||
| 32.1 to 49.9 kg: 750 mg | ||
| ≥ 50.0 kg: 900 mg maximum | ||
Patients Who May Receive the Combined Regimen
This regimen is recommended for otherwise healthy persons 12 years and older who have latent M. tuberculosis infection and other risk factors for tuberculosis (i.e., recent exposure to contagious tuberculosis, conversion from negative to positive on an indirect skin test, and radiographic findings of healed pulmonary tuberculosis). Persons with human immunodeficiency virus (HIV) infection who are otherwise healthy and not taking antiretroviral medications are also included in this recommendation, as well as those who do not fall into these categories but who are unlikely to complete nine months of daily isoniazid therapy because of practical reasons (e.g., persons living in correctional facilities or homeless shelters). Persons who have underlying illnesses associated with tuberculosis (e.g., diabetes mellitus) or illnesses that could decrease the tolerability of the combined therapy should be considered on an individual basis. Ultimately, the choice between isoniazid monotherapy and combined isoniazid and rifapentine therapy depends on patient and physician preferences.
The preferred regimen for children two to 11 years of age is daily isoniazid therapy for nine months because there is insufficient evidence to support the use of combined therapy in this age group. However, combined isoniazid and rifapentine may be considered if completing daily isoniazid for nine months is unlikely, or if the child has a high risk of developing tuberculosis (e.g., recent M. tuberculosis infection in a preschool-aged child).
Patients Who Should Not Receive the Combined Regimen
Patients who should not receive combined isoniazid and rifapentine therapy include the following: children younger than two years, because the safety of this therapy has not been established; persons with HIV infection taking antiretroviral therapy, because the drug interactions have not been studied; pregnant women or those who may become pregnant during the course of treatment, because safety in pregnancy is unknown; and patients who have latent M. tuberculosis infection that is presumed to be resistant to isoniazid or rifampin.
Precautions
Treatment of latent M. tuberculosis infection should be avoided in persons with active tuberculosis because of the risks of partial treatment and drug resistance. Radiographic findings of presumed healed tuberculosis should be examined to confirm that it is not still active. Additionally, patients with HIV infection also are more likely to show extrapulmonary tuberculosis or pulmonary tuberculosis with normal findings on chest radiography.
Adverse effects associated with rifapentine include the reddening of secretions, such as urine and tears, as well as the staining of contact lenses. Uncommon adverse effects include neutropenia and increased serum concentration of liver enzymes. Hypersensitivity reactions, although rare, may induce symptoms such as fever, headache, dizziness, musculoskeletal pain, petechiae, purpura, and pruritus. Unless carefully monitored, rifapentine should not be used in patients taking medications with narrow therapeutic ranges (e.g., methadone, warfarin [Coumadin]). Women taking rifapentine and hormonal contraceptives should add or switch to a barrier method.
Directly observed therapy is needed because missed doses or incorrect dosing intervals could alter the safety and effectiveness of medications. Workers trained to directly observe the administration of therapy should use a symptom checklist for adverse effects and report any problems to a physician. Monthly clinical assessments that include a physical examination and inquiries about adverse effects are recommended. If patients report adverse effects, therapy should be withheld while the cause is being determined. Baseline and subsequent tests should be performed in select patients (Table 2). Severe adverse effects of treatment should be reported to the CDC and to the U.S. Food and Drug Administration's MedWatch program at http://www.fda.gov/medwatch.

| Educate patients to seek medical attention upon the first symptom of a possible adverse effect | |
| Assess patients upon the first sign or symptom of a possible adverse effect | |
| Conduct monthly interview and brief physical examination for the findings of a treatment-associated adverse effect (e.g., icterus, tenderness of the liver, rash) | |
| Obtain baseline hepatic chemistry blood tests (at least alanine transaminase) for patients with specific conditions (e.g., human immunodeficiency virus infection, liver disorders, within three months postpartum, regular alcohol use) | |
| Consider baseline hepatic chemistry blood test for older patients on an individual basis, especially for those taking medications for chronic medical conditions | |
| Obtain blood tests at subsequent clinical encounters for patients whose baseline testing is abnormal and for others at risk of liver disease | |
| Discontinue combined isoniazid and rifapentine therapy if a serum transaminase concentration is five times the upper limit of normal or greater even in the absence of symptoms, or three times the upper limit of normal or greater in the presence of symptoms | |
| Monitor patients for drug hypersensitivity reactions, particularly hypotension or thrombocytopenia | |
| Severe condition (e.g., hypotension requiring intravenous fluid support): discontinue combined isoniazid and rifapentine therapy; provide supportive medical care | |
| Mild to moderate condition (e.g., dizziness treated with rest or oral fluids): provide conservative management of constitutional symptoms; continue clinical and laboratory monitoring; consider option to continue treatment under observation | |
