
Am Fam Physician. 2012;86(6):501-508
Related: U.S. Preventive Services Task Force Recommendation Statement and Putting Prevention Into Practice.
Author disclosure: No relevant financial affiliations to disclose.
The American Cancer Society (ACS), the American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology (ASCP) recently published updated recommendations for the early detection of cervical cancer.1 This topic was last reviewed in 2002. The guidelines are based on a systematic evidence review. In an effort to build consensus, draft recommendations were posted online for public comment, and 25 organizations participated in a guideline symposium where they discussed and voted on each recommendation.
As with the 2002 updates, the new ACS/ASCCP/ASCP guidelines suggest a movement toward decreasing excess screening and incorporating emerging technologies when clinically feasible. The 2012 guidelines state that women younger than 21 years should not be screened for cervical cancer, regardless of age of sexual initiation or other risk factors.1 Cervical cancer is rare in young women, and screening may not prevent cancer in this age group. Human papillomavirus (HPV) infections are common and generally transient, and most cervical lesions regress without intervention. Overtreatment leads to net harm in young women.2
For women 21 to 29 years of age, the new guidelines recommend screening with cytology alone every three years. Annual screening of women at any age is not recommended because it results in only a slightly greater reduction in cancer risk but twice the number of colposcopies compared with screening every three years.1 The incidence of cervical cancer is three per 1,000 women among those who are screened annually, and increases to four to six per 1,000 with a two-year screening interval and five to eight per 1,000 with a three-year interval. There is no statistically significant difference in cancer reduction between a two- and three-year screening interval, and the shorter two-year screening interval results in a 40 percent increase in the number of colposcopies.1,3
Women 30 to 65 years of age should be screened with cytology and HPV testing (i.e., co-testing) every five years, which is the preferred approach, or cytology alone every three years, which is acceptable.1 Co-testing leads to increased detection of prevalent cervical intraepithelial neoplasia grade 3 (CIN3), with a concomitant decrease in cancer or CIN grade 3 or higher (CIN3+) detected in subsequent rounds of screening. This permits a longer interval between screenings, with cancer incidence rates similar to or lower than screening with cytology alone at shorter intervals, as well as fewer colposcopies. A 2011 study showed that the risk of invasive cancer three years after a negative Papanicolaou (Pap) smear result was 0.018 percent (95% confidence interval, 0.008 to 0.041 percent); five years after a negative co-test, the risk of cancer was 0.016 percent (95% confidence interval, 0.003 to 0.072 percent).4 In addition, co-testing every five years is associated with approximately 17 percent fewer colposcopies compared with cytology alone every three years.3 Finally, co-testing increases the detection of adenocarcinomas, which are often missed with cytology alone.4–6
The new guidelines recommend against screening in women older than 65 years who have evidence of adequate negative screening in the past 10 years and no history of CIN2+ within the past 20 years.1 In well-screened women older than 65 years, CIN2+ prevalence is low and cervical cancer is rare. Most new HPV infections in women older than 65 years clear spontaneously. The potential harm outweighs the small potential benefit of screening women in this age group.
Vaginal cuff smear following hysterectomy for benign reasons appears to be a common clinical practice that is lacking in proven effectiveness and that accounts for untold medical costs and patient discomfort. Despite this, more than 10 million women continue to have this unnecessary screening.7
Vaginal cancer is rare, with an age-specific incidence similar to or less than that of other cancers for which screening is not performed, such as breast cancer in men. Abnormal vaginal cytology is seldom of clinical importance. The ACS/ASCCP/ASCP guidelines reaffirm that women of any age who have no history of CIN2+ and who undergo hysterectomy with removal of the cervix for benign conditions should not be screened for vaginal cancer using any modality.1 The Good Stewardship Working Group of the National Physicians Alliance has placed Pap smears in these women on its top-five list of tests not to perform in family medicine.8
ASCCP-sponsored consensus recommendations for the management of abnormal cytology results were published in 2006.9 The 2012 ACS/ASCCP/ASCP guidelines provide additional guidance for the treatment of women with discordant co-testing results.1 Women whose HPV test results are positive and cytology results are negative should repeat co-testing in 12 months (first option) or undergo immediate HPV genotype-specific testing for types 16 or 16/18 (second option). The short-term risk of CIN3 in these women is far less than when an HPV-positive test is associated with atypical squamous cells of undetermined significance (ASCUS) or with low-grade squamous intraepithelial lesions, and most transient infections clear within 12 months. Therefore, these women should not be referred directly for colposcopy. Genotype-specific HPV tests that are positive for types 16 or 16/18 are associated with clinically relevant short-term risk of CIN3 or cancer, and colposcopy is recommended in those cases.
Women with ASCUS on cytology and a negative HPV test result should continue routine screening as per age-specific guidelines. The risk of precancerous lesions in these women does not warrant increased surveillance over women who have negative cytology and negative HPV test results. The absolute risk of CIN3+ in women with ASCUS on cytology and negative HPV test results is very low (less than 2 percent).4,10–12
Family physicians are encouraged to fully review the ACS/ASCCP/ASCP cervical cancer screening guidelines,1 the U.S. Preventive Services Task Force (USPSTF) screening guidelines,13 and the ASCCP consensus recommendations.9 Table 1 compares the ACS/ASCCP/ASCP guidelines with the new recommendations from the USPSTF.1,13 Recommended screening practices should not change based on HPV vaccination status, pending further evidence on the duration of protection and absolute risk reduction among vaccinated women. The biggest gain in reducing cervical cancer incidence and mortality can be achieved by increasing screening rates among women who have not been screened or who have not been screened regularly.14 Family physicians should seek to identify and screen these women.
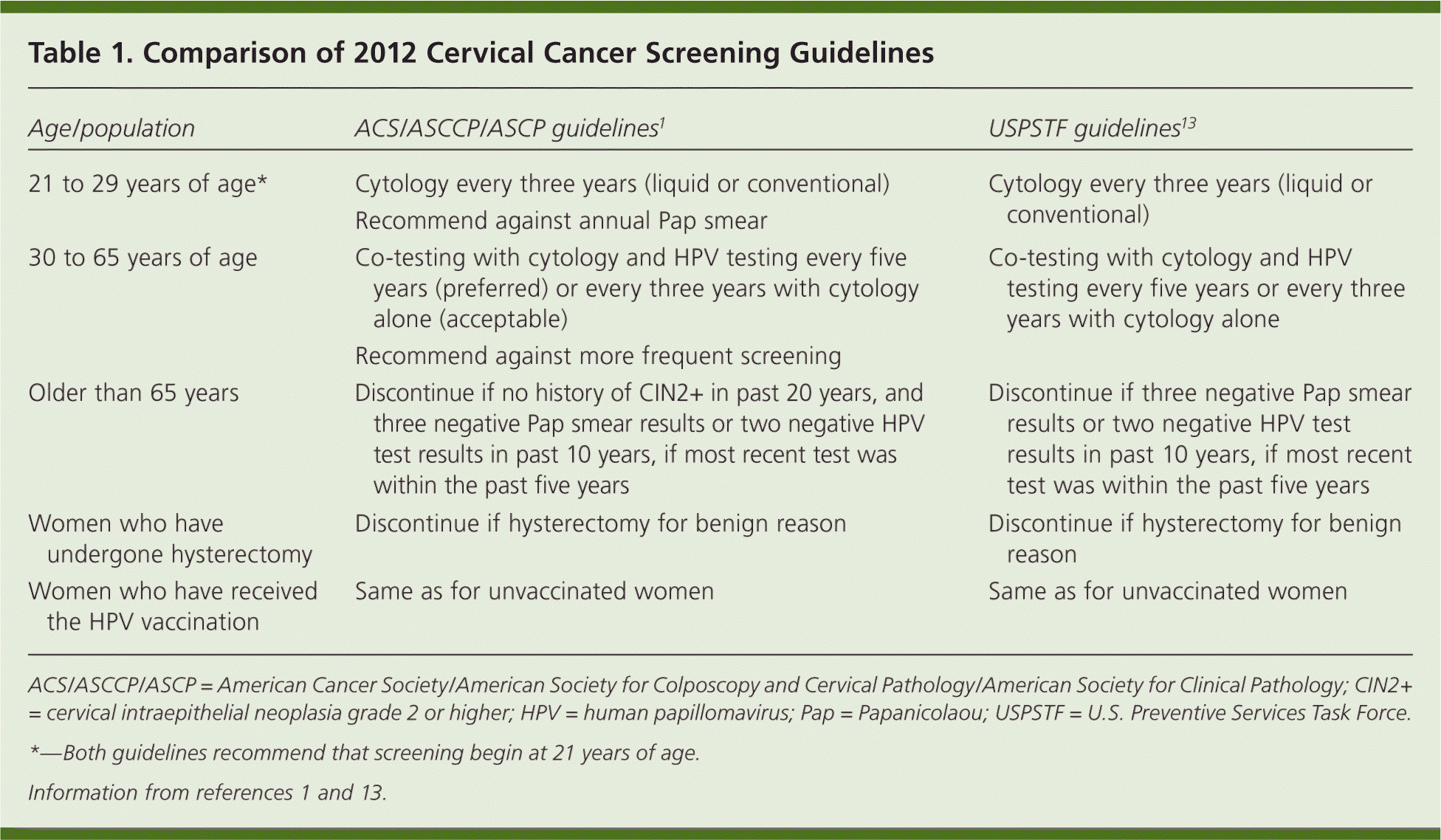
| Age/population | ACS/ASCCP/ASCP guidelines1 | USPSTF guidelines13 |
|---|---|---|
| 21 to 29 years of age* |
|
|
| 30 to 65 years of age |
|
|
| Older than 65 years |
|
|
| Women who have undergone hysterectomy |
|
|
| Women who have received the HPV vaccination |
|
|
editor's note: Dr. Saslow is the lead author of the ACS/ASCCP/ASCP guidelines on screening for the prevention and early detection of cervical cancer.
