
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2012;86(11):1027-1034
Patient information: See related handout on hepatitis A, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Hepatitis A is a common viral illness worldwide, although the incidence in the United States has diminished in recent years as a result of extended immunization practices. Hepatitis A virus is transmitted through fecal-oral contamination, and there are occasional outbreaks through food sources. Young children are usually asymptomatic, although the likelihood of symptoms tends to increase with age. Most patients recover within two months of infection, although 10 to 15 percent of patients will experience a relapse in the first six months. Hepatitis A virus does not usually result in chronic infection or chronic liver disease. Supportive care is the mainstay of treatment. The Centers for Disease Control and Prevention and the American Academy of Pediatrics recommend routine vaccination of all children 12 to 23 months of age, as well as certain vulnerable populations. Hepatitis A vaccine is also recommended for most cases of postexposure prophylaxis, although immunoglobulin is an acceptable alternative in some situations.
Hepatitis A is one of the world's most common viral infections. Although most patients recover within two months, the disease can produce significant morbidity, which can be largely prevented with appropriate immunization strategies.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Hepatitis A vaccine should be given to all children 12 to 23 months of age. | C | 7, 37 | — |
| Persons who have recently been exposed to hepatitis A virus and have not been immunized should receive a single dose of single-antigen hepatitis A vaccine, or immunoglobulin as an alternative. | C | 8 | Healthy persons between 12 months and 40 years of age should receive the hepatitis A vaccine. |
| Children younger than 12 months, persons 40 years and older, immunocompromised persons, and persons with chronic liver disease or for whom the vaccine is contraindicated, should receive immunoglobulin. | |||
| All susceptible persons traveling outside of the United States, with the exceptions of Australia, New Zealand, Canada, western Europe, and Japan, should receive at least one dose of hepatitis A vaccine as soon as travel is considered. | C | 8 | Older adults, persons who are immunocompromised, and persons who have chronic liver disease or other chronic conditions, should receive immunoglobulin and hepatitis A vaccine. |
| Immunoglobulin can provide protection at usual doses for three months to travelers younger than 12 months, persons not wishing to receive the vaccine, or persons who are allergic to the vaccine. | |||
| Hepatitis A vaccine should be routinely offered to patients at high risk for infection. | C | 1, 2, 7–9 | See Table 1 for risk groups. |
Epidemiology
Hepatitis A virus (HAV) is an enveloped RNA agent classified as a picornavirus that can produce symptomatic or asymptomatic infection in humans. It is the cause of approximately one-half of all reported cases of viral hepatitis in the United States, although the prevalence of HAV infections has declined by 92 percent since the introduction of a vaccine in 1995.1 In 2009, it was estimated that more than 21,000 cases of hepatitis A occurred in the United States, and 1.4 million cases occurred worldwide.2
The virus is shed in the stool, and is primarily spread by food contaminated with fecal matter. Hepatitis A can also be contracted from contaminated water, personal contact (such as being in the same household with a person who has the virus, or through children at day care centers), sexual contact (especially in men who have sex with men), and illicit drug use3–5 (Table 16–9 ). Approximately 55 percent of cases do not have an identifiable risk factor.10
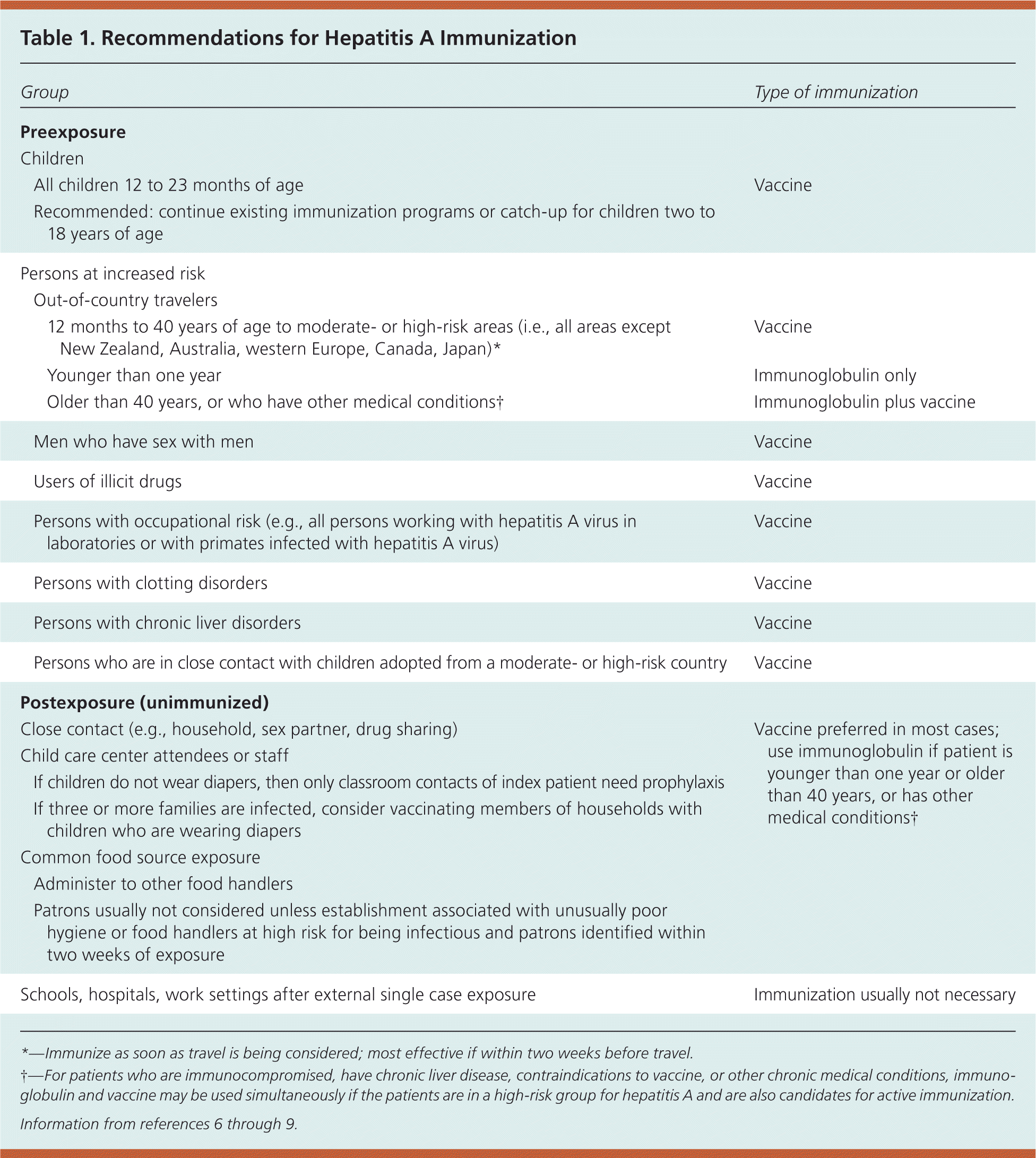
| Group | Type of immunization | ||
|---|---|---|---|
| Preexposure | |||
| Children | |||
| All children 12 to 23 months of age | Vaccine | ||
| Recommended: continue existing immunization programs or catch-up for children two to 18 years of age | |||
| Persons at increased risk | |||
| Out-of-country travelers | |||
| Vaccine | ||
| Immunoglobulin only | ||
| Immunoglobulin plus vaccine | ||
| Men who have sex with men | Vaccine | ||
| Users of illicit drugs | Vaccine | ||
| Persons with occupational risk (e.g., all persons working with hepatitis A virus in laboratories or with primates infected with hepatitis A virus) | Vaccine | ||
| Persons with clotting disorders | Vaccine | ||
| Persons with chronic liver disorders | Vaccine | ||
| Persons who are in close contact with children adopted from a moderate- or high-risk country | Vaccine | ||
| Postexposure (unimmunized) | |||
| Close contact (e.g., household, sex partner, drug sharing) | Vaccine preferred in most cases; use immunoglobulin if patient is younger than one year or older than 40 years, or has other medical conditions† | ||
| Child care center attendees or staff | |||
| If children do not wear diapers, then only classroom contacts of index patient need prophylaxis | |||
| If three or more families are infected, consider vaccinating members of households with children who are wearing diapers | |||
| Common food source exposure | |||
| Administer to other food handlers | |||
| Patrons usually not considered unless establishment associated with unusually poor hygiene or food handlers at high risk for being infectious and patrons identified within two weeks of exposure | |||
| Schools, hospitals, work settings after external single case exposure | Immunization usually not necessary | ||
Clinical Presentation
The infection typically has an abrupt onset following an incubation period of approximately 28 days (range: 15 to 50 days).11 Signs and symptoms of infection can include nausea, vomiting, diarrhea, dark urine, jaundice, fever, headache, weight loss, and abdominal pain, as well as a loss of desire for cigarette smoking or alcohol. The likelihood of symptoms increases with age. Most children younger than six years are asymptomatic and can be a source of infection to others during the brief period they are infected, mainly by the fecal-oral route.12 Jaundice appears in more than 70 percent of older children and adults infected with HAV. Hepatomegaly and splenomegaly may be present. Peak infectivity can be present two weeks before and at least one week after symptoms begin, although in some cases with recurrent illness, it may last longer. Acute illness typically does not last more than two months.1 There is no chronic viral shedding and no chronic stage of the disease, although recurrences, acute fulminant hepatitis, and other complications may occur.
Evaluation
Given the broad differential diagnosis, HAV cannot be diagnosed solely on clinical grounds and cannot be distinguished from other types of hepatitis without laboratory testing. Clinical suspicion is increased if there has been any exposure to raw vegetables or fruit, other uncooked or undercooked foods and drinking water that is not sanitized, or exposure to a person with known HAV infection. There have been no recent reports of shellfish as the source of HAV outbreaks in the United States, although this has been common in other parts of the world.13
The clinical presentation of hepatitis A makes it difficult to differentiate from other types of acute viral hepatitis because symptoms overlap with many other gastrointestinal and febrile conditions. The differential diagnosis includes other viral infections, drugs, toxins, bacterial infections, parasitic infections, and autoimmune hepatitis (Table 2).14 [ corrected] Physicians should be aware of risk groups for hepatitis A as opposed to other types of viral hepatitis in establishing the differential diagnosis (Table 16–9 ).
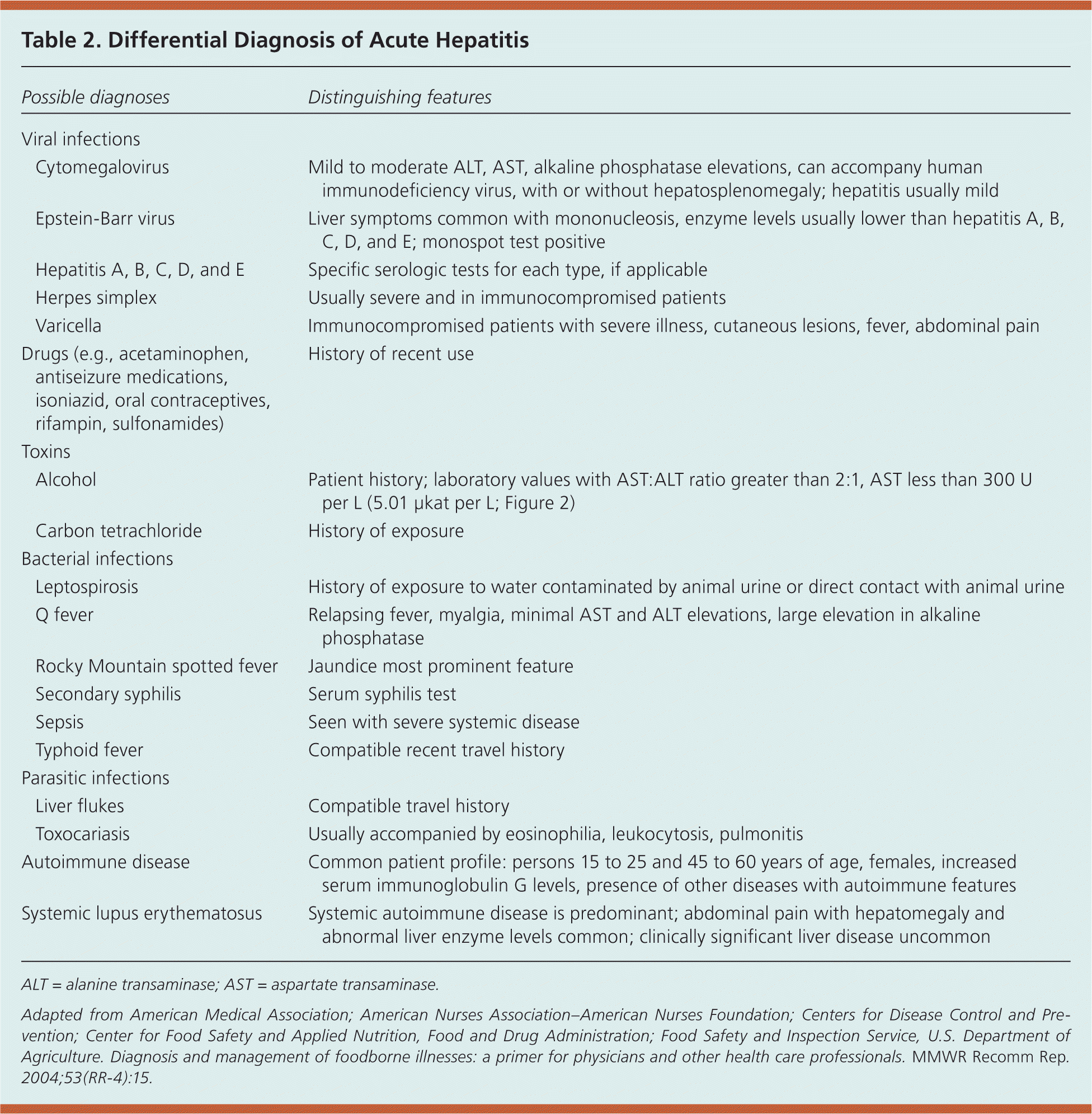
| Possible diagnoses | Distinguishing features | |
|---|---|---|
| Viral infections | ||
| Cytomegalovirus | Mild to moderate ALT, AST, alkaline phosphatase elevations, can accompany human immunodeficiency virus, with or without hepatosplenomegaly; hepatitis usually mild | |
| Epstein-Barr virus | Liver symptoms common with mononucleosis, enzyme levels usually lower than hepatitis A, B, C, D, and E; monospot test positive | |
| Hepatitis A, B, C, D, and E | Specific serologic tests for each type, if applicable | |
| Herpes simplex | Usually severe and in immunocompromised patients | |
| Varicella | Immunocompromised patients with severe illness, cutaneous lesions, fever, abdominal pain | |
| Drugs (e.g., acetaminophen, antiseizure medications, isoniazid, oral contraceptives, rifampin, sulfonamides) | History of recent use | |
| Toxins | ||
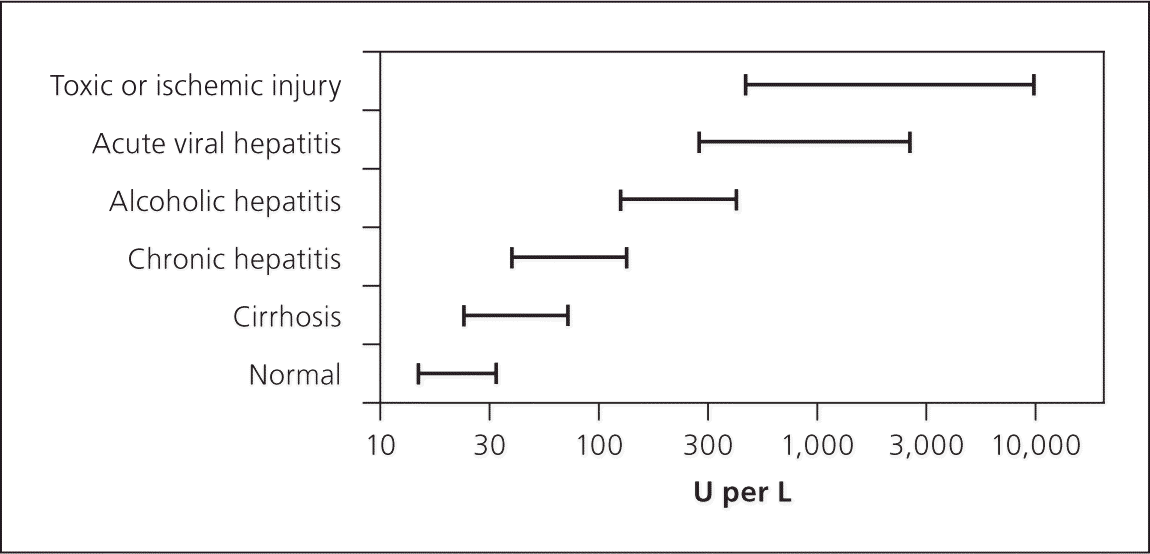
| Alcohol | Patient history; laboratory values with AST:ALT ratio greater than 2:1, AST less than 300 U per L (5.01 μkat per L; Figure 2) | |
| Carbon tetrachloride | History of exposure | |
| Bacterial infections | ||
| Leptospirosis | History of exposure to water contaminated by animal urine or direct contact with animal urine | |
| Q fever | Relapsing fever, myalgia, minimal AST and ALT elevations, large elevation in alkaline phosphatase | |
| Rocky Mountain spotted fever | Jaundice most prominent feature | |
| Secondary syphilis | Serum syphilis test | |
| Sepsis | Seen with severe systemic disease | |
| Typhoid fever | Compatible recent travel history | |
| Parasitic infections | ||
| Liver flukes | Compatible travel history | |
| Toxocariasis | Usually accompanied by eosinophilia, leukocytosis, pulmonitis | |
| Autoimmune disease | Common patient profile: persons 15 to 25 and 45 to 60 years of age, females, increased serum immunoglobulin G levels, presence of other diseases with autoimmune features | |
| Systemic lupus erythematosus | Systemic autoimmune disease is predominant; abdominal pain with hepatomegaly and abnormal liver enzyme levels common; clinically significant liver disease uncommon | |
Laboratory findings in persons who are symptomatic show marked elevation in serum transaminase, total and direct bilirubin, and alkaline phosphatase levels. With any type of viral hepatitis, the alanine transaminase (ALT) level is typically higher than the aspartate transaminase (AST) level, and the range for both is usually between 500 and 5,000 U per L (8.35 to 83.5 μkat per L; Figure 115). Elevations in transaminase levels occur before bilirubin elevation, but may coincide with the onset of clinical illness (Figure 214 ).
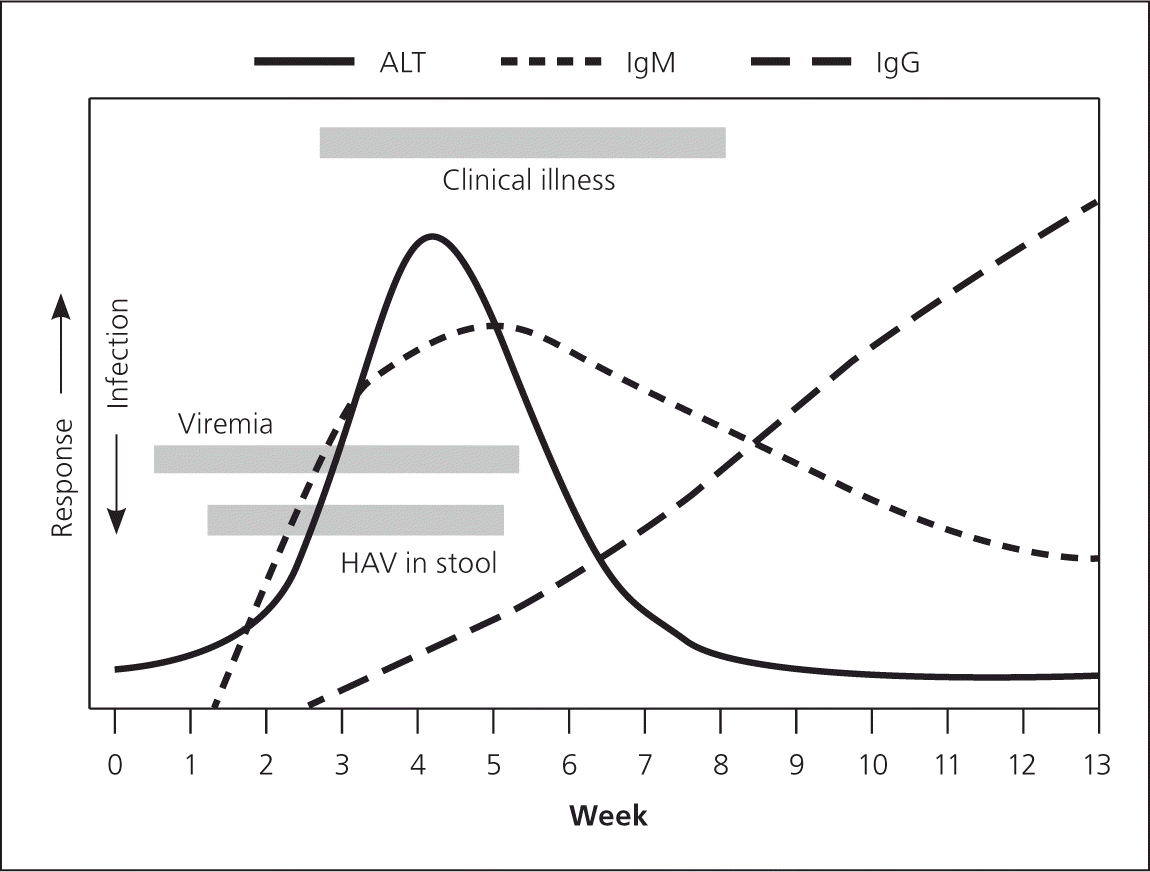
Diagnosis is normally made by the detection of serum immunoglobulin M (IgM) anti-HAV antibodies. Tests for IgM anti-HAV can distinguish acute hepatitis A from other forms of hepatitis, and its sensitivity and specificity are greater than 95 percent.6 The IgM anti-HAV test becomes positive within five to 10 days, but typically does not detect the lower concentrations that exist four to six months following an acute infection. IgM anti-HAV can also be detected in persons who recently received the hepatitis A vaccine.5 False-positive results do occur; thus, the test should be reserved for persons who have symptoms.16 Total anti-HAV (IgM and immunoglobulin G) remains positive after infection or immunization for a patient's lifetime, and is useful only in identifying unimmunized patients at risk. HAV begins to be excreted in the stool shortly after the ALT level begins to increase and just before IgM is detectable. Clinical illness typically begins shortly after; thus, HAV may be transmitted before symptoms occur 7 (Figure 214 ).
Complications
Hepatitis A is self-limiting in most cases; complications are more common in adults older than 50 years.1 Between 10 and 15 percent of persons who are infected will have a relapse up to six months after the acute illness has resolved, but there is no development of chronic hepatitis A. It is important to understand that the virus may be excreted during a relapse and can be transmitted during this time. Fulminant hepatitis is uncommon, and caused approximately 100 deaths each year in the United States in the pre-vaccine era.1,17,18 The risk is increased if there is underlying liver disease, such as hepatitis B or C,19,20 or if there is coinfection with more than one HAV genotype at the same time.21
Rarely, vasculitis, arthritis, thrombocytopenia, acute pancreatitis, aplastic or autoimmune hemolytic anemia, Guillain-Barré syndrome, acute renal failure, or pericarditis may occur in patients with HAV infection.18,22–27 There is a case report of acute renal failure and acute pericarditis occurring in a patient with HAV infection.22
Approach to the Patient
Only supportive treatment is available for the routine case of hepatitis A. Bed rest is usually advised, and patients should not return to work or school until fever and jaundice have subsided. Age-appropriate treatment for nausea and diarrhea should be provided. Patients should avoid alcohol, but may eat normally as tolerated. Fulminant hepatitis A occasionally requires emergency liver transplant.28 Pregnant women who contract hepatitis A have an increased incidence of gestational complications and preterm labor that should be treated accordingly.29
Immunization
ACTIVE IMMUNIZATION
The licensure of vaccines for hepatitis A in 1995 and 1996 opened the way for the eventual prevention of this disease in the United States. Originally, emphasis was placed on immunizing children living in communities with the highest incidence of disease, and of certain other groups at higher risk. By 2005, most cases of hepatitis A were occurring outside of the areas previously recommended for routine vaccination. In 2006, the Centers for Disease Control and Prevention (CDC) made significant changes by recommending that all children 12 to 23 months of age be immunized.7
The vaccines available in the United States are inactivated. The single-antigen vaccines are Havrix and Vaqta. The combination vaccine Twinrix contains the HAV and the hepatitis B virus antigens. Twinrix contains a smaller dose of HAV, and is indicated for active immunization only.
The vaccine should be administered intramuscularly in the deltoid muscle, and two formulations are available depending on the age of the patient (Table 3).7,30 Immunity is probably lifelong, and is present in nearly 100 percent of immunocompetent patients one month after receiving the recommended two doses.7 Studies indicate that adequate antibody levels could be present for 25 years or longer in adults, and 14 years or longer in children.31 There are no data evaluating the need for revaccination following the two-dose schedule in immunocompetent patients. Patients with human immunodeficiency virus infection may have lower response rates. Patients with chronic liver disease, individuals 40 years and older, and illicit drug users may also have slightly lower rates, although the evidence is not strong.32–34
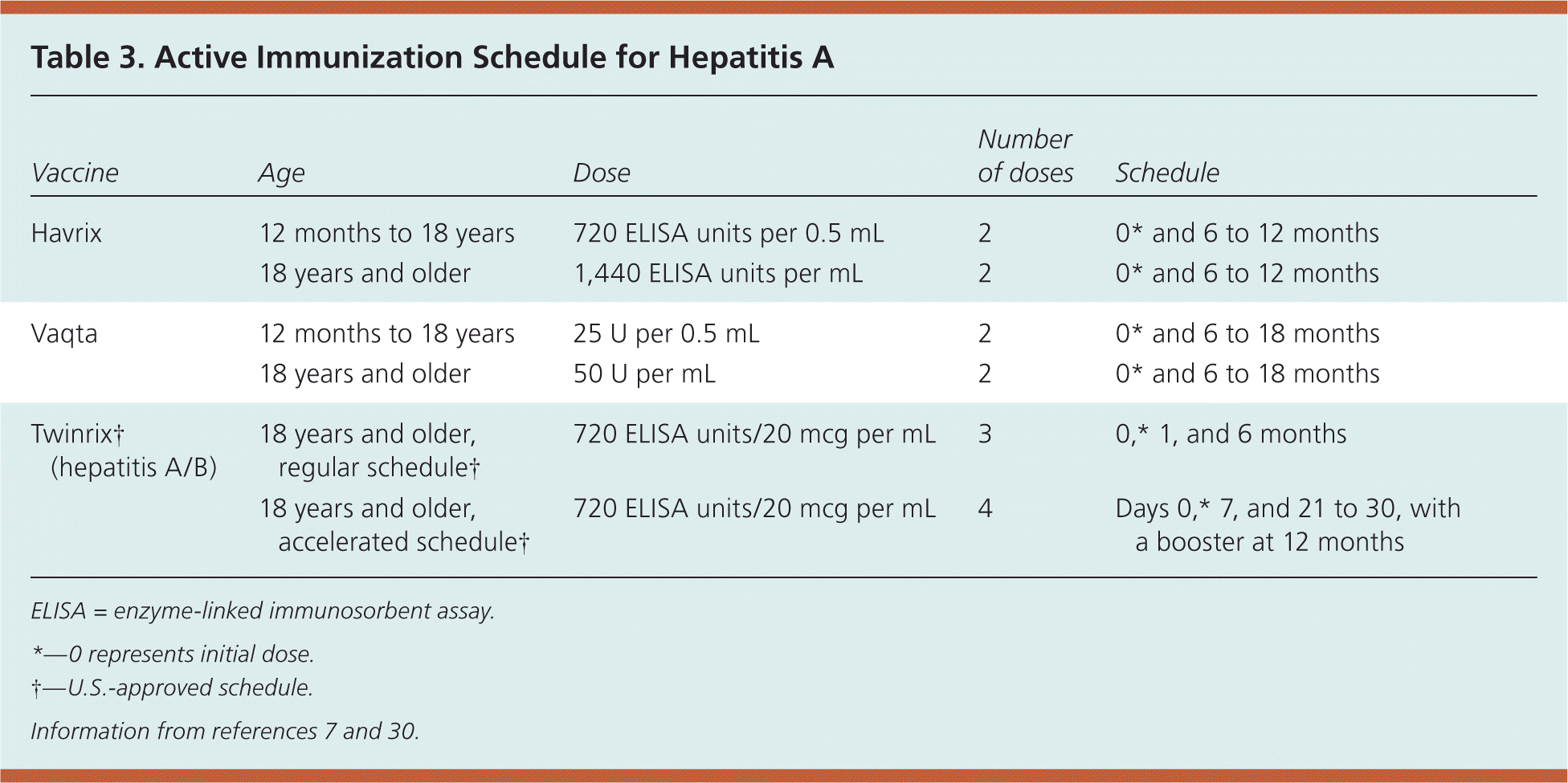
| Vaccine | Age | Dose | Number of doses | Schedule |
|---|---|---|---|---|
| Havrix | 12 months to 18 years | 720 ELISA units per 0.5 mL | 2 | 0* and 6 to 12 months |
| 18 years and older | 1,440 ELISA units per mL | 2 | 0* and 6 to 12 months | |
| Vaqta | 12 months to 18 years | 25 U per 0.5 mL | 2 | 0* and 6 to 18 months |
| 18 years and older | 50 U per mL | 2 | 0* and 6 to 18 months | |
| Twinrix† (hepatitis A/B) | 18 years and older, regular schedule† | 720 ELISA units/20 mcg per mL | 3 | 0,* 1, and 6 months |
| 18 years and older, accelerated schedule† | 720 ELISA units/20 mcg per mL | 4 | Days 0,* 7, and 21 to 30, with a booster at 12 months |
All forms of the hepatitis A vaccine can be given with other vaccines without affecting the immune response or causing an increase in adverse events. Side effects are rare, and include tenderness at the injection site, headache, and malaise.35 The vaccine should not be given to persons with a history of a serious reaction to a previous injection of hepatitis A vaccine.
If an individual is from an area with high endemicity of hepatitis A, or from certain population groups with a high incidence, it may be cost-effective to pretest for immunity. The total anti-HAV should be used for pre-testing. There are no current recommendations for posttesting.
PASSIVE IMMUNIZATION
Immunoglobulin provides passive transfer of hepatitis A antibody. It can be used for preexposure prophylaxis (and in certain cases, for postexposure prophylaxis), and is protective for varying periods of time depending on the dose, although hepatitis A vaccine is most commonly recommended. Immunoglobulin is most commonly used in this country for preexposure prophylaxis for certain travelers or under specific circumstances for postexposure prophylaxis (Table 430,36 ). It is 80 to 90 percent protective for up to five months, depending on the dose administered.36
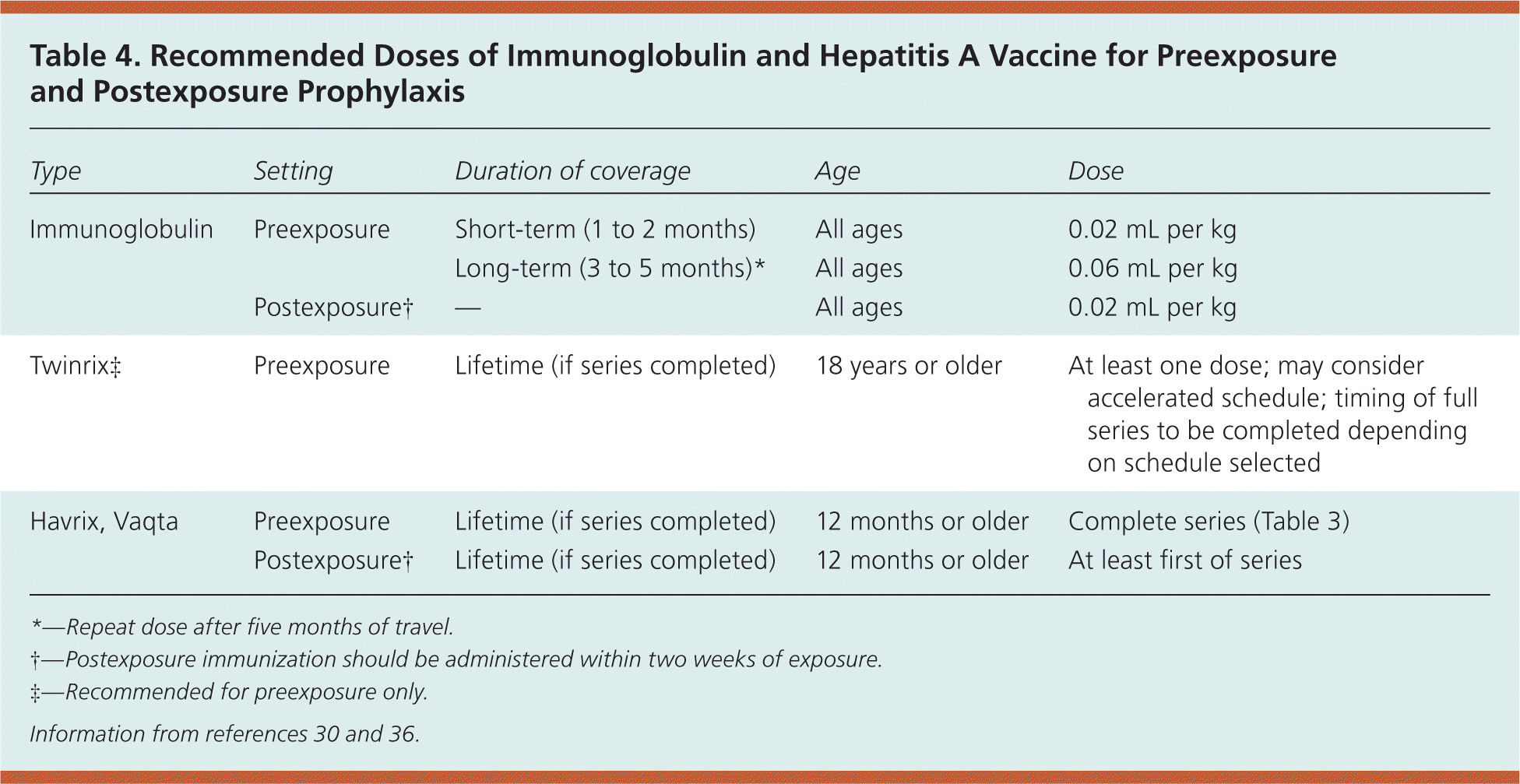
| Type | Setting | Duration of coverage | Age | Dose |
|---|---|---|---|---|
| Immunoglobulin | Preexposure | Short-term (1 to 2 months) | All ages | 0.02 mL per kg |
| Long-term (3 to 5 months)* | All ages | 0.06 mL per kg | ||
| Postexposure† | — | All ages | 0.02 mL per kg | |
| Twinrix‡ | Preexposure | Lifetime (if series completed) | 18 years or older | At least one dose; may consider accelerated schedule; timing of full series to be completed depending on schedule selected |
| Havrix, Vaqta | Preexposure | Lifetime (if series completed) | 12 months or older | Complete series (Table 3) |
| Postexposure† | Lifetime (if series completed) | 12 months or older | At least first of series |
RECOMMENDATIONS FOR PREEXPOSURE PROTECTION
Children. Recommendations from the CDC and the American Academy of Pediatrics state that all children should receive hepatitis A vaccine as part of routine childhood immunizations, beginning the series between 12 and 23 months of age. Children older than 23 months who have not been immunized previously should be considered for routine immunization.7,37
Individuals at Increased Risk. All persons at high risk for hepatitis A infection should routinely be offered the hepatitis A vaccine. Travelers to most areas outside of the United States, with the exceptions of Australia, New Zealand, Canada, western Europe, and Japan, should be immunized. For optimal protection, vaccination with a single-dose hepatitis A vaccine should be undertaken as soon as travel is anticipated.8 Protection is probably present at least by two weeks after the first injection, and completion of the series is recommended for long-term protection. If the patient is 40 years or older, is immunocompromised, has chronic liver disease, or plans on departing in less than two weeks, then simultaneous administration of immunoglobulin (0.02 mL per kg) with the vaccine may be considered.8 If an individual does not want the vaccine, is younger than 12 months, or for some other reason cannot take the vaccine, then a single dose of immunoglobulin as listed above will be protective for up to three months. If travel is expected to exceed two months, then the dose should be increased to 0.06 mg per kg, and repeated after the estimated travel date has exceeded five months.8
If desired, an accelerated schedule of Twinrix can be used in which three doses can be given over 21 days (at days 0, 7, and 21 to 30), with a fourth dose at one year. This regimen can be used as a protective measure for patients 18 years and older, and has been shown to produce an adequate immune response for hepatitis A and B antigens30 (Table 37,30 ).
Men who have sex with men, users of illicit drugs, persons with occupational risks (such as health care professionals and those who work with HAV-infected animals), persons with clotting-factor disorders, persons with chronic liver disease, persons 18 years and older (Twinrix Junior is not yet available in the United States) who travel to areas of high endemicity for HAV and hepatitis B virus, and other persons at high risk, could all be vaccinated with Twinrix.8
The CDC also recommends that persons in close contact with children who are being adopted from a country outside of those excepted under the travel recommendations should be immunized. This includes household contacts and babysitters.9
There is no strong evidence to support routine immunization of persons who handle food. Immunization may be part of a program to limit the potential spread of hepatitis A during an outbreak.
Postexposure Prophylaxis
If a healthy individual has recently been exposed to HAV, has not been immunized previously, and is between 12 months and 40 years of age, a single dose of single-antigen vaccine is the preferred prophylaxis according to the CDC, although immunoglobulin can be used.8 The vaccine or immunoglobulin should be given within two weeks of exposure, because effectiveness beyond two weeks is not known. If the vaccine is contraindicated, the patient is younger than one year or is 40 years or older, the patient is immunocompromised, or the patient has chronic liver disease, then immunoglobulin is recommended (0.02 mL per kg). If the vaccine is recommended for other risk factors, then immunoglobulin and vaccine can be administered in separate sites simultaneously, and the remaining dose should be administered according to the prescribed schedule.
Postexposure prophylaxis should be offered to all previously unvaccinated persons who are in close contact with a person who has hepatitis A, including sex partners and persons who are sharing illicit drugs with an infected patient. Postexposure prophylaxis should also be offered to staff members and attendees of child care centers in settings with one or more cases of hepatitis A in a child or employee, or if two or more cases exist in the households of attendees; and to food handlers under certain conditions30,36 (Table 16–9 ). If an outbreak (defined as hepatitis A in three or more families) occurs, consideration should be given to immunizing members of households that have children in diapers. Prophylaxis is not usually necessary for individuals in contact with a person who has hepatitis A when a single case occurs in an elementary or secondary school or office setting if the source of infection is outside that setting, or when a patient with hepatitis A is admitted to a hospital.8
Prevention Practices
Prophylaxis of vulnerable populations and children through active or passive immunization is the most important prevention practice. Heating foods to 185°F (85°C) for one minute, use of a 1:100 solution of household bleach, handwashing, and avoiding contact with uncooked foods, are all techniques that may reasonably decrease the likelihood of hepatitis A transmission. Other than thorough cooking, there is no reliable disinfection technique for shellfish that will decrease transmission.6,13
Data Sources: We searched for English-only articles on hepatitis A published between 2003 and 2011 via Medline for diagnosis, clinical course, complications, treatment, transmission, protection, and immunization, restricted to human participants. We also reviewed the Agency for Health-care Research and Quality Evidence Reports, U.S. Preventive Services Task Force reports, Institute for Clinical Systems Improvement, National Guideline Clearinghouse, and the Cochrane Database of Systematic Reviews. An evidence summary of Essential Evidence Plus was also reviewed for relevant articles and information. Search date: January 2011.
