
Am Fam Physician. 2012;86(12):1144-1148
Author disclosure: No relevant financial affiliations to disclose.
Linagliptin (Tradjenta) is a dipeptidyl-peptidase-4 (DPP-4) inhibitor labeled for the treatment of type 2 diabetes mellitus. Similar to sitagliptin (Januvia) and saxagliptin (Onglyza), linagliptin delays the breakdown of endogenous incretin hormones such as glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide. These hormones, when secreted in response to food intake, stimulate postmeal insulin secretion, inhibit glucagon release, improve satiety, and slow gastric emptying.1,2 Linagliptin can be used alone or in combination with metformin (Glucophage), sulfonylureas, pioglitazone (Actos), or insulin.
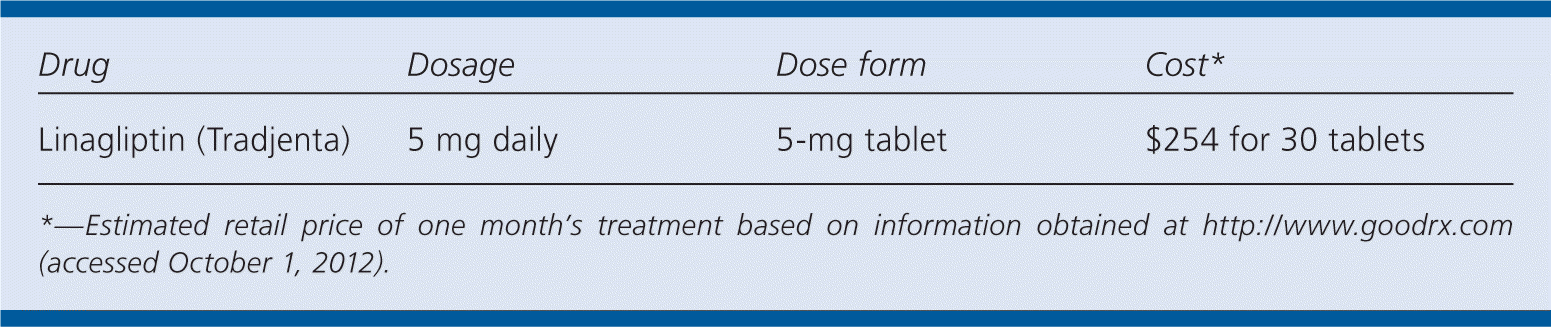
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Linagliptin (Tradjenta) | 5 mg daily | 5-mg tablet | $254 for 30 tablets |
SAFETY
Few severe adverse effects have been associated with linagliptin. When used alone or with metformin or pioglitazone, linagliptin has an incidence of hypoglycemia comparable to that of placebo.1–4 Hypoglycemia is more common with linagliptin (22.9 percent) than with placebo (14.8 percent) when added to the combination of metformin and a sulfonylurea.5 A reduction in sulfonylurea or insulin dosage may be needed to decrease the risk of hypoglycemia when linagliptin is added to these agents.2 As with other DPP-4 inhibitors, pancreatitis can occur, although infrequently, with linagliptin therapy; eight cases were reported in clinical trials involving 4,687 patients (0.2 percent).2
Patients who have renal dysfunction do not require a dosage reduction with linagliptin, unlike with the other DPP-4 inhibitors. Linagliptin has no clinically relevant drug interactions with metformin, pioglitazone, digoxin, simvastatin (Zocor), or warfarin (Coumadin). Alternative agents for glucose control should be used in patients receiving strong inducers of cytochrome P450 3A4 or P-glycoprotein, such as rifampin. Linagliptin is a U.S. Food and Drug Administration pregnancy category B drug.2
TOLERABILITY
Overall, linagliptin is well tolerated, and reports of adverse effects are similar to those with placebo.1–5 Patients taking linagliptin had slightly higher rates of back pain (9.1 versus 8.4 percent), arthralgia (8.1 versus 6.1 percent), and headache (6.4 versus 5.2 percent) than patients treated with a sulfonylurea.2 Treatment discontinuation because of adverse effects was infrequent (1.2 to 2.9 percent).1–4 Linagliptin does not affect renal function, body weight, or waist circumference.1,3
EFFECTIVENESS
No studies have determined linagliptin's effect on diabetes-related complications and mortality. It reduces levels of A1C by 0.4 to 0.7 percent,2,3 fasting plasma glucose (FPG) by 20.5 to 23.4 mg per dL (1.14 to 1.30 mmol per L), and two-hour postprandial glucose by 58.4 mg per dL (3.24 mmoL per L).1,2 Adding linagliptin to metformin, sulfonylureas, or pioglitazone will reduce A1C levels by an additional 0.4 to 0.6 percent,2–4 with slightly smaller reductions in FPG.2–5 When used in combination with insulin (with or without metformin, pioglitazone, or both), linagliptin reduces A1C levels by an additional 0.65 percent and FPG by an additional 11 mg per dL (0.61 mmol per L).2 Linagliptin has not been compared head-to-head with other DPP-4 inhibitors.
PRICE
A 30-day supply of linagliptin (5-mg tablets) will cost about $250. This cost is comparable to that of other DPP-4 inhibitors. Generic metformin and sulfonylureas are available for less than $5 per month through most retail pharmacy discount programs.
SIMPLICITY
Linagliptin is available in 5-mg tablets taken once daily with or without food. It is contra-indicated in patients with a history of hypersensitivity to the drug.2
Bottom Line
Linagliptin reduces A1C levels to a lesser extent than first-line therapy (metformin) and is significantly more expensive than metformin and sulfonylureas. Importantly, its ability to affect diabetes-related morbidity and mortality is not known. As with other DPP-4 inhibitors, linagliptin may be useful as an add-on therapy for patients with type 2 diabetes who require additional agents to achieve A1C goals. Linagliptin affects measures of diabetes similarly to the other DPP-4 inhibitors, saxagliptin and sitagliptin, but does not require dosage adjustments in patients with renal dysfunction.
