
Am Fam Physician. 2013;87(4):274-278
A more recent article on breast cancer screening is available.
Related editorial: Screening Mammograpy: The Goal Is Changing.
Author disclosure: No relevant financial affiliations to disclose.
Breast cancer is the most common non–skin cancer and the second leading cause of cancer death in North American women. Mammography is the only screening test shown to reduce breast cancer–related mortality. There is general agreement that screening should be offered at least biennially to women 50 to 74 years of age. For women 40 to 49 years of age, the risks and benefits of screening should be discussed, and the decision to perform screening should take into consideration the individual patient risk, values, and comfort level of the patient and physician. Information is lacking about the effectiveness of screening in women 75 years and older. The decision to screen women in this age group should be individualized, keeping the patient's life expectancy, functional status, and goals of care in mind. For women with an estimated lifetime breast cancer risk of more than 20 percent or who have a BRCA mutation, screening should begin at 25 years of age or at the age that is five to 10 years younger than the earliest age that breast cancer was diagnosed in the family. Screening with magnetic resonance imaging may be considered in high-risk women, but its impact on breast cancer mortality is uncertain. Clinical breast examination plus mammography seems to be no more effective than mammography alone at reducing breast cancer mortality. Teaching breast self-examination does not improve mortality and is not recommended; however, women should be aware of any changes in their breasts and report them promptly.
Breast cancer is the most common non–skin cancer and the second leading cause of cancer death in North American women. In the United States, there were an estimated 230,480 new cases of invasive breast cancer and an estimated 39,970 deaths attributed to it in 2011.1 Worldwide, approximately 458,400 deaths were attributed to breast cancer.2
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Teaching breast self-examination does not reduce mortality and is not recommended. | A | 4, 5, 18, 20, 21 | — |
| Clinical breast examination is an option for women in all risk categories, but should not replace screening mammography. | C | 8–10, 21 | The U.S. Preventive Services Task Force states that there is insufficient evidence to support clinical breast examination.18 |
| Annual or biennial screening mammography should be offered to average-risk women 50 to 74 years of age. | A | 8–10, 18, 20–22 | There is general agreement to screen women 50 to 70 years of age. |
| For average-risk women 40 to 49 years of age, the risks and benefits of mammography are closely balanced. The decision to perform screening mammography should take into consideration the individual patient risk, values, and comfort level of the patient and physician. | B | 18–21 | Other organizations maintain their strong support to start routine screening at 40 years of age.8–10,22 |
| Annual or biennial screening mammography can be offered to average-risk women older than 74 years. This decision should be individualized, keeping the patient's life expectancy, functional status, and goals of care in mind. | C | 8, 10 | — |
In the United States and other industrialized countries, mortality rates from breast cancer have been declining by 2.2 percent per year since 1990,2 largely because of the increased use of screening mammography and greater use of adjuvant therapies.3 Although screening mammography has contributed significantly to reducing breast cancer mortality, ongoing controversy remains about the age at which routine screening should start and stop, as well as the optimal frequency of screening. This article presents current evidence and recommendations for breast cancer screening, and provides a reasonable approach to screening women with mammography based on expected benefits and individual patient risk. The roles of clinical breast examination, breast self-examination, magnetic resonance imaging, and other screening tools will also be reviewed.
Screening Modalities
BREAST SELF-EXAMINATION
Although it is a common practice, teaching breast self-examination does not reduce breast cancer mortality and may increase false-positive rates. Two large randomized trials, one in China involving more than 266,000 women and the other in Russia involving more than 120,300 women, did not demonstrate a mortality benefit from teaching breast self-examination.4,5 A review of eight other studies did not show a benefit for the rate of breast cancer diagnosis, the tumor size or stage, or the rate of death from breast cancer.6
Instead of breast self-examination, some organizations recommend encouraging women 20 years and older to recognize the normal appearance and feel of their breasts, without using any systematic examination technique.7–10 The goal of breast self-awareness is for women to promptly report any changes in their breasts to their primary care physician.7 Although there are no studies to support this recommendation, the number of times that women find lumps that lead to a breast cancer diagnosis warrants educating them to recognize and report changes in their breasts.
CLINICAL BREAST EXAMINATION
In a study of 39,405 women 50 to 59 years of age, clinical breast examination (CBE) alone was compared with CBE plus mammography, and after 13 years of follow-up the mortality rate was the same in each group.11,12 A review of controlled trials and case-control studies that included CBE as a screening modality estimated CBE sensitivity and specificity to be 54 and 94 percent, respectively.13 A subsequent study found that CBE plus mammography had greater sensitivity than mammography alone, but also had a higher false-positive rate.14 A literature review performed for the U.S. Preventive Services Task Force (USPSTF) concluded that the effectiveness of CBE has not been established in well-designed large trials.15
MAMMOGRAPHY
When to Begin Screening. Although there is general agreement that screening mammography should be offered routinely to women 50 to 74 years of age, there are conflicting guidelines for its use in women 40 to 49 years of age. In 2009, the USPSTF recommended against routine screening mammography in women younger than 50 years, based on the analysis of closely balanced benefits and harms.18 The USPSTF noted that the rates of false-positive results in young women were nearly double those in women 50 years and older; that the number needed to screen for women 39 to 49 years of age to prevent one breast cancer death was much higher than that for women 50 to 59 and 60 to 69 years of age (1,904, 1,339, and 377, respectively); and that the risks of overdiagnosis (e.g., ductal carcinoma in situ that may not grow or become invasive) and overtreatment were additional potential harms.18
After the USPSTF recommendations were published, a large Swedish cohort study reported 16-year results comparing breast cancer mortality between women 40 to 49 years of age who were invited to undergo screening (study group) and women in the same age group who were not invited (control group).19 Screening every 18 to 24 months was associated with a 26 to 29 percent relative risk reduction in breast cancer mortality, with a number needed to screen of 1,252 over 10 years. However, this was not a randomized study, and the authors acknowledged the possibility of selection bias caused by differences between the study and control groups.
Although a number of major organizations support the USPSTF recommendations,20,21 many professional societies and organizations in the United States have maintained their strong support for systematic screening in women older than 40 years.8–10,22 The USPSTF subsequently updated its recommendation by stating that “the decision to start regular, biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient's values regarding specific benefits and harms.”18
When to Stop Screening. The optimal age at which to stop routine breast cancer screening is uncertain. There is no information from clinical trials about the effectiveness of screening mammography in women older than 74 years, and the USPSTF has concluded that the evidence is insufficient to assess the additional benefits and harms of screening mammography in women 75 years and older.18 The American Cancer Society and the National Comprehensive Cancer Network recommend that as long as an older woman is in good health and remains a candidate for breast cancer treatment if necessary, she should continue to be screened.8,10
Screening Intervals. Most guidelines recommend screening every one to two years in women 50 years and older. For women 40 to 49 years of age who desire screening, the American College of Obstetricians and Gynecologists (ACOG) recommends annual mammography.9 ACOG's previous guideline recommended routine mammography every one to two years starting at 40 years of age, and then annually beginning at 50 years. The comparatively rapid growth of breast cancers in women younger than 50 years and the potential for early detection to reduce mortality in this age group were two of the main reasons cited for the change.9
A recent modeling study found that a woman's age, breast density, family history, and history of breast biopsy affect the cost-effectiveness of screening mammography. Biennial screening for most women 50 to 74 years of age is cost-effective based on a cost per quality-adjusted life-year threshold of $100,000 or less.23
Digital vs. Film Mammography. Studies comparing digital with film mammography have produced conflicting results. However, the Digital Mammographic Imaging Screening Trial, which involved 50,000 asymptomatic women 40 years and older, showed that the overall accuracy of film and digital mammography was similar, and that digital mammography is more sensitive than film in women younger than 50 years, in those who are pre-menopausal, and in those with dense breast tissue.24
Limitations of the Evidence. Although screening mammography reduces breast cancer mortality, the magnitude of that effect remains uncertain, making it difficult to weigh against the potential harms. A Cochrane review acknowledged that screening is likely to reduce breast cancer mortality, but estimated a relative risk reduction of only 15 percent.25 In addition, the authors also noted that screening led to 30 percent overdiagnosis and overtreatment. This means that for every 2,000 women screened over 10 years, one will have her life prolonged and 10 healthy women will be treated unnecessarily. Furthermore, more than 200 women will experience prolonged psychological distress related to false-positive findings. Most of the randomized trials of screening mammography were conducted decades ago, when effective treatment options for breast cancer were limited, and some studies suggest that improvements in treatment may have reduced the magnitude of benefit to be gained from screening.26
ULTRASONOGRAPHY
There are no data that document the value of ultrasound screening alone. A study comparing mammography alone with mammography plus ultrasonography in high-risk women with dense breasts found that the addition of ultrasonography substantially increased the rate of cancer detection, but at the cost of increased false-positive results (10.4 percent compared with 4.4 percent for mammography alone).27 The most important use of breast ultrasonography is in the evaluation of suspicious lesions found during screening mammography and of those found by physical examination but not detected by mammography.
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging has greater sensitivity than mammography and can provide additional information compared with mammography.28,29 Prospective studies, including a large international study, suggest that this modality should be used as a screening tool in women at high risk because of dense breast tissue, family history, or BRCA1 and BRCA2 mutations.30–34 However, because of a lack of standard procedure, performance, and interpretation, the results from one institution may not be reproducible in another.35 The American Cancer Society and the National Comprehensive Cancer Network recommend the addition of magnetic resonance imaging to mammography for women with a known BRCA mutation, those with a first-degree relative who has a BRCA mutation, and those with a lifetime risk of 20 percent or more.8,10 They recommend that screening begin at 25 to 30 years of age, and continue for as long as a woman is in good health, although the exact timing and screening interval remain unclear.
OTHER SCREENING MODALITIES
Although scintimammography, positron emission tomography, ductal lavage, and thermography have been considered as possible tools for breast cancer screening, none are currently used because of cost, impracticality, or lack of validation in prospective trials.36
Practical Approach to Breast Cancer Screening
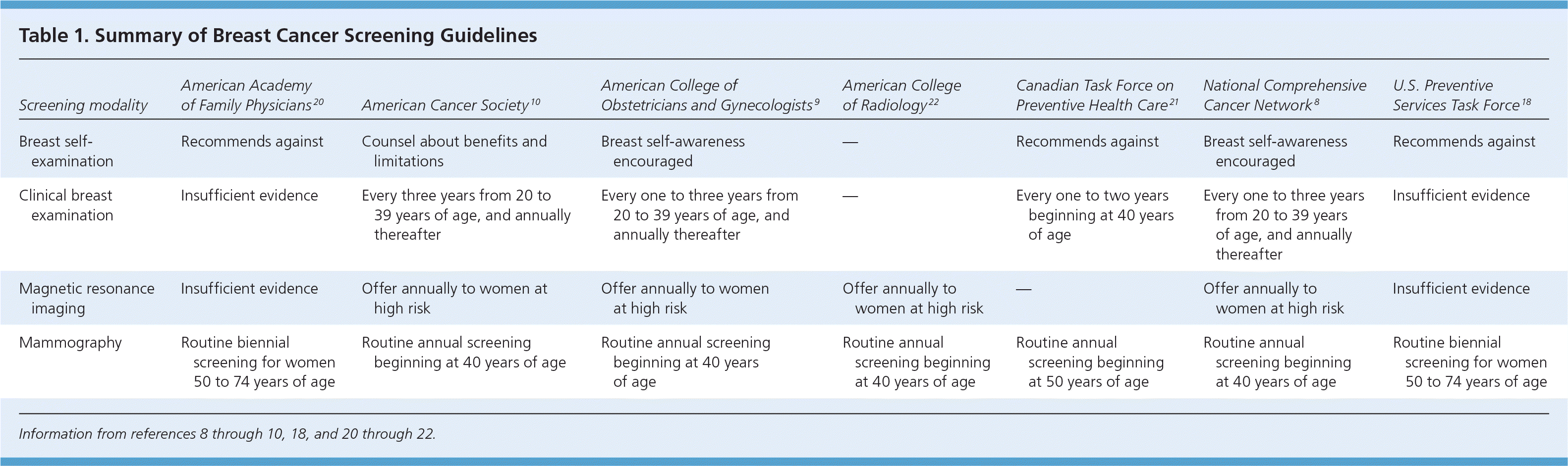
| Screening modality | American Academy of Family Physicians20 | American Cancer Society10 | American College of Obstetricians and Gynecologists9 | American College of Radiology22 | Canadian Task Force on Preventive Health Care21 | National Comprehensive Cancer Network8 | U.S. Preventive Services Task Force18 |
|---|---|---|---|---|---|---|---|
| Breast self-examination | Recommends against | Counsel about benefits and limitations | Breast self-awareness encouraged | — | Recommends against | Breast self-awareness encouraged | Recommends against |
| Clinical breast examination | Insufficient evidence | Every three years from 20 to 39 years of age, and annually thereafter | Every one to three years from 20 to 39 years of age, and annually thereafter | — | Every one to two years beginning at 40 years of age | Every one to three years from 20 to 39 years of age, and annually thereafter | Insufficient evidence |
| Magnetic resonance imaging | Insufficient evidence | Offer annually to women at high risk | Offer annually to women at high risk | Offer annually to women at high risk | — | Offer annually to women at high risk | Insufficient evidence |
| Mammography | Routine biennial screening for women 50 to 74 years of age | Routine annual screening beginning at 40 years of age | Routine annual screening beginning at 40 years of age | Routine annual screening beginning at 40 years of age | Routine annual screening beginning at 50 years of age | Routine annual screening beginning at 40 years of age | Routine biennial screening for women 50 to 74 years of age |
The following approach is recommended based on broad consensus within the guidelines to the care of individual patients:
For women 50 to 74 years of age, physicians should offer screening mammography annually or biennially.
For women 40 to 49 years of age, risk stratification is an important component of assessing the potential benefits of breast cancer screening. The most commonly used risk-prediction model, the Breast Cancer Risk Assessment Tool, is available on the National Cancer Institute Web site (http://www.cancer.gov/bcrisktool/).37 The variables used to calculate five-year and lifetime risk of breast cancer are listed in Table 2.37 For women at high risk of breast cancer (i.e., a lifetime risk greater than 20 to 25 percent), or with known BRCA1 or BRCA2 mutations, screening mammography should be recommended. For women at average risk (lifetime risk less than 15 percent) or moderate risk (15 to 20 percent), the harms and benefits of mammography should be discussed, and the decision to perform mammography should be determined by individual patient risk, values, and comfort level. For average-risk women older than 74 years, screening mammography can be considered depending on the patient's health, life expectancy, functional status, and goals of care.
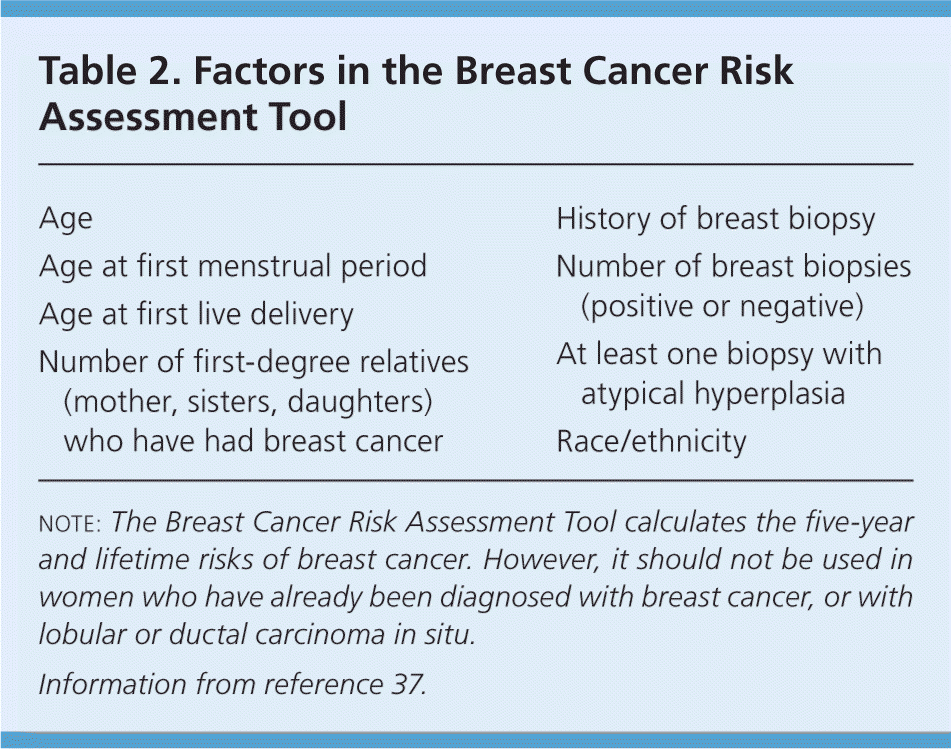
| Age |
| Age at first menstrual period |
| Age at first live delivery |
| Number of first-degree relatives (mother, sisters, daughters) who have had breast cancer |
| History of breast biopsy |
| Number of breast biopsies (positive or negative) |
| At least one biopsy with atypical hyperplasia |
| Race/ethnicity |
Data Sources: A search of electronic databases, including the Cochrane Library, the Agency for Healthcare Research and Quality clinical guidelines and evidence reports, Academic Search Complete, and PubMed, was completed using the key terms breast cancer, breast cancer screening, early detection, mammography, risk reduction, and combinations of these terms. The search yielded meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates were limited to January 1, 2000, through August 30, 2011. Also searched were the Canadian Task Force on Preventive Health Care, the National Guideline Clearinghouse, and the U.S. Preventive Services Task Force. Lists of key references were also searched in an iterative fashion. Search dates: August 2011 to July 2012.
