
Am Fam Physician. 2013;87(7):494-501
Author disclosure: No relevant financial affiliations to disclose.
Risks of diagnostic imaging include cancer from radiation exposure and nephrogenic systemic fibrosis. The increase in volume of imaging between 1980 and 2006 has led to a sixfold increase in annual per capita radiation exposure. It is predicted that 2 percent of future cancers will be caused by radiation from computed tomography (CT) exposure. Gadolinium contrast media should be avoided in patients with stage 4 or 5 chronic kidney disease because of the risk of nephrogenic systemic fibrosis. Appropriate use of imaging based on guidelines for specific clinical conditions can reduce these risks. Although noncontrast CT of the head is needed to rule out bleeding in patients with suspected stroke within the first three hours of symptom onset, diffusion-weighted imaging with magnetic resonance of the head and neck is superior to CT within three to 24 hours of symptom onset. Headache merits neuroimaging in special circumstances only. Sestamibi radioisotope has less radiation than thallium for myocardial perfusion imaging. Use of intravenous contrast media with abdominopelvic CT significantly increases the diagnostic accuracy for appendicitis. Cholescintigraphy has better discrimination to diagnose acute cholecystitis than CT in patients with equivocal ultrasonography results. Limited three-view intravenous urography is recommended in pregnancy to evaluate urolithiasis if initial ultrasonography findings are negative or equivocal. Given that many asymptomatic adults have abnormal findings on lumbar spine magnetic resonance imaging, this modality generally should not be performed for nonspecific chronic low back pain in the absence of red flags. Whole body scanning is not supported by current evidence.
Increased use of imaging procedures and the resultant escalation of exposure to ionizing radiation has sparked greater awareness and concern for public safety.1 Adverse effects of diagnostic imaging include nephrogenic systemic fibrosis and future cancer risks from radiation exposure. Judicious use of imaging based on the clinical presentation and the evidence behind various modalities can help reduce these risks.
Volume of Imaging Use
In 2006, 380 million radiologic procedures (including 67 million computed tomography [CT] scans) and 18 million nuclear medicine procedures were performed in the United States.2 To highlight the disproportionate use, U.S. patients received approximately one-half of all nuclear medicine procedures worldwide while making up only 4.6 percent of the global population.2 The volume represents a sixfold increase (from 0.5 to 3.0 mSv [millisieverts]) in annual per capita radiation exposure from 1980 to 2006.2 The terms “sievert” and “rem” (which reflect the amount of absorbed radiation multiplied by a tissue weighting factor) are preferred over the older terms “gray” or “rad” to measure biologic damage.3 During the past 30 years, the natural background radiation dose (2.4 mSv annual per capita) has remained constant.2
Radiation Measurement and Limits
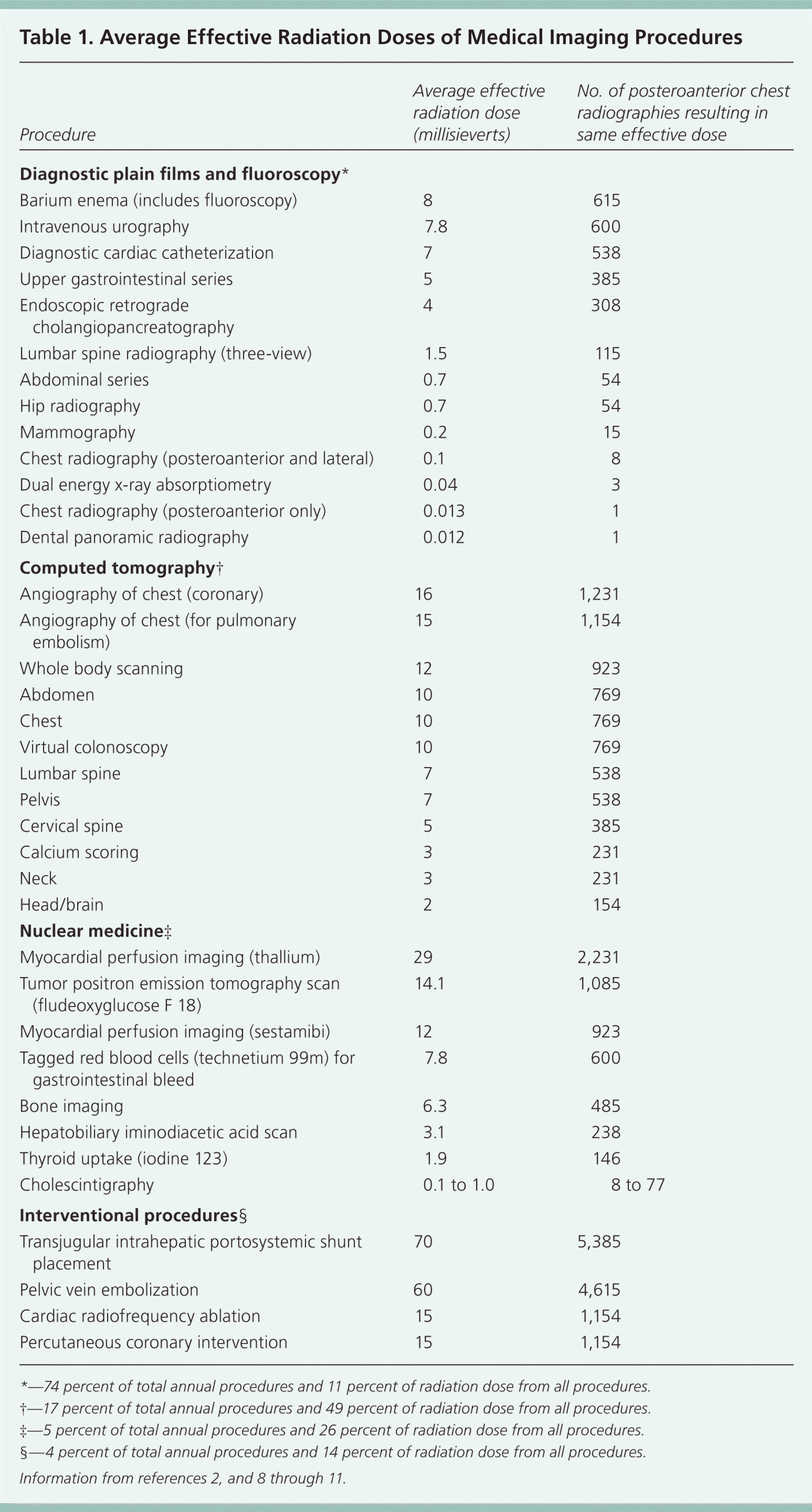
To put radiation risk into perspective, it is instructive to highlight recommended exposure limits. The mean lethal dose of radiation that will kill 50 percent of the population within 60 days if untreated is estimated at 3,500 to 4,000 mSv.4 Occupational limits set by the U.S. Department of Energy allow for up to 50 mSv per year in adult radiology workers.5 Pregnant workers are restricted to 5 mSv per year; it is recommended that minors and the general public not exceed 1 mSv per year.5 Pregnant patients exposed to less than 100 mSv are considered at low risk of fetal malformation.6 Above 500 mSv, fetal damage can be substantial depending on the dose and estimated gestational stage.6,7 Because of the varying individual requirements in patient care, annual absolute limits for diagnostic imaging have not been widely established (Table 12,8–11 ).
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Gadolinium-based contrast agents should be avoided in patients with stage 4 or 5 chronic kidney disease because of the risk of nephrogenic systemic fibrosis. | C | 14–16 | U.S. Food and Drug Administration boxed warning issued in September 2010 |
| When available, diffusion-weighted magnetic resonance angiography of the head and neck is more accurate than CT angiography for the diagnosis of acute ischemic stroke when performed without delay within three to 24 hours of symptom onset, and it better predicts future functionality. | C | 21, 25, 26 | Sensitivity of diffusion-weighted imaging to exclude cerebral hemorrhage is insufficient to omit head CT |
| Ultrasonography is the preferred initial imaging modality for suspected acute cholecystitis or cholelithiasis. | C | 36, 38 | CT commonly, and mistakenly, performed as first-line imaging |
| In the absence of red flags, magnetic resonance imaging is not indicated in patients with chronic nonspecific low back pain. | C | 42–47 | See Table 3 for red flags |
| Whole body scanning should be avoided for the detection of preclinical disease. | C | 48–51 | Definitive harm and unsubstantiated benefit |
| Procedure | Average effective radiation dose (millisieverts) | No. of posteroanterior chest radiographies resulting in same effective dose |
|---|---|---|
| Diagnostic plain films and fluoroscopy* | ||
| Barium enema (includes fluoroscopy) | 8 | 615 |
| Intravenous urography | 7.8 | 600 |
| Diagnostic cardiac catheterization | 7 | 538 |
| Upper gastrointestinal series | 5 | 385 |
| Endoscopic retrograde cholangiopancreatography | 4 | 308 |
| Lumbar spine radiography (three-view) | 1.5 | 115 |
| Abdominal series | 0.7 | 54 |
| Hip radiography | 0.7 | 54 |
| Mammography | 0.2 | 15 |
| Chest radiography (posteroanterior and lateral) | 0.1 | 8 |
| Dual energy x-ray absorptiometry | 0.04 | 3 |
| Chest radiography (posteroanterior only) | 0.013 | 1 |
| Dental panoramic radiography | 0.012 | 1 |
| Computed tomography† | ||
| Angiography of chest (coronary) | 16 | 1,231 |
| Angiography of chest (for pulmonaryembolism) | 15 | 1,154 |
| Whole body scanning | 12 | 923 |
| Abdomen | 10 | 769 |
| Chest | 10 | 769 |
| Virtual colonoscopy | 10 | 769 |
| Lumbar spine | 7 | 538 |
| Pelvis | 7 | 538 |
| Cervical spine | 5 | 385 |
| Calcium scoring | 3 | 231 |
| Neck | 3 | 231 |
| Head/brain | 2 | 154 |
| Nuclear medicine‡ | ||
| Myocardial perfusion imaging (thallium) | 29 | 2,231 |
| Tumor positron emission tomography scan (fludeoxyglucose F 18) | 14.1 | 1,085 |
| Myocardial perfusion imaging (sestamibi) | 12 | 923 |
| Tagged red blood cells (technetium 99m) for gastrointestinal bleed | 7.8 | 600 |
| Bone imaging | 6.3 | 485 |
| Hepatobiliary iminodiacetic acid scan | 3.1 | 238 |
| Thyroid uptake (iodine 123) | 1.9 | 146 |
| Cholescintigraphy | 0.1 to 1.0 | 8 to 77 |
| Interventional procedures§ | ||
| Transjugular intrahepatic portosystemic shunt placement | 70 | 5,385 |
| Pelvic vein embolization | 60 | 4,615 |
| Cardiac radiofrequency ablation | 15 | 1,154 |
| Percutaneous coronary intervention | 15 | 1,154 |
Cancer Risk
Increasing recognition of future cancer risk from radiation exposure was illustrated in a 2009 study showing that 2 percent of all future cancer cases will likely come from previous CT exposure, resulting in approximately 15,000 deaths annually.12 A 13-fold difference existed between the lowest and highest amounts of radiation emitted for each study protocol, even within the same institutions, which emphasizes the hazard of underestimating the cumulative risk for an individual patient.13
Nephrogenic Systemic Fibrosis Risk
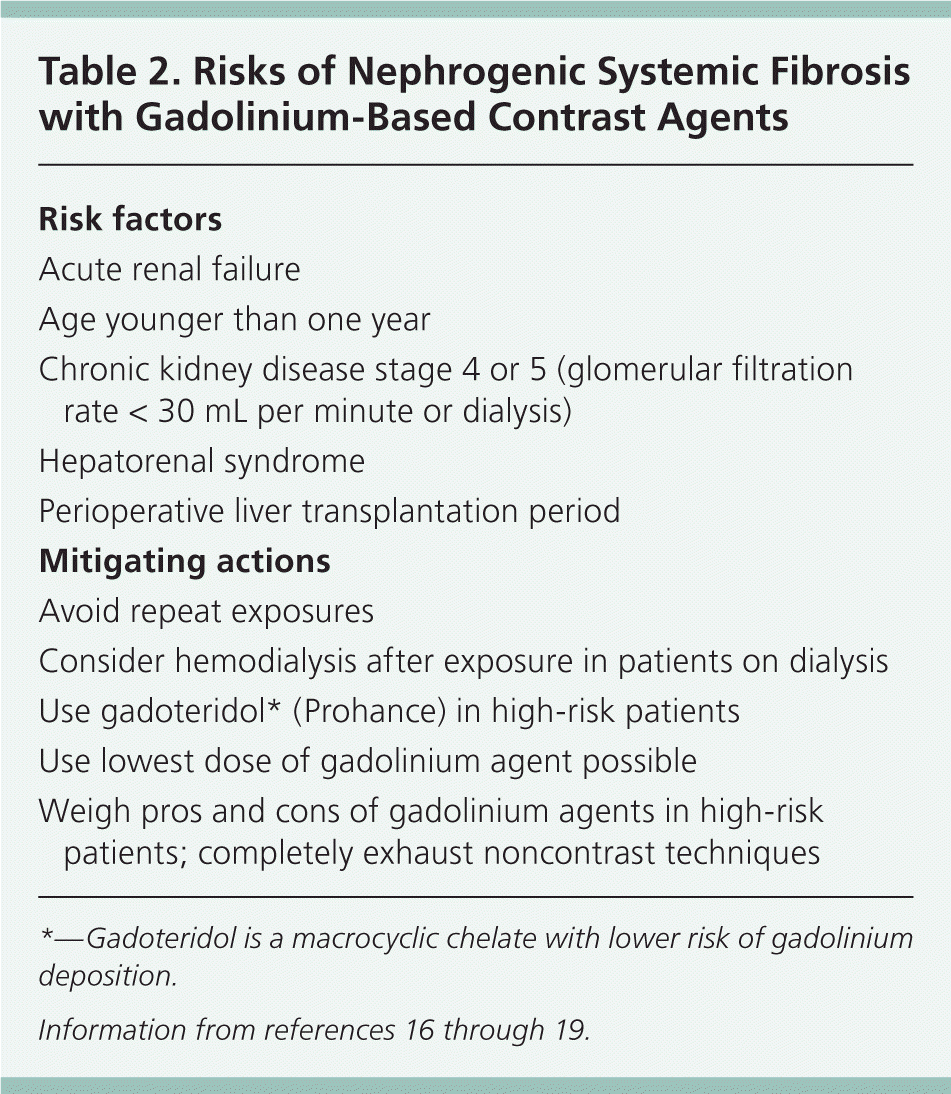
Nephrogenic systemic fibrosis is characterized by scleroderma-like tissue changes in the skin, internal organs, eyes, and blood vessels. Gadolinium-based magnetic resonance imaging (MRI) contrast agents are associated with the incidence of nephrogenic systemic fibrosis.14 A U.S. Food and Drug Administration boxed warning applies to all gadolinium agents, but linear, ionically charged structures are at highest risk of deposition of toxic gadolinium.15,16 Examples include gadodiamide (Omniscan), gadopentetate (Magnevist), and gadoversetamide (Optimark), which are contraindicated in patients with acute kidney injury or severe chronic kidney disease (stage 4 or 5)—the groups at highest risk of nephrogenic systemic fibrosis.15 Other factors that should be considered before gadolinium use are listed in Table 2.16–19 No cases of nephrogenic systemic fibrosis have been reported for chronic kidney disease stages 1 through 3.16
| Risk factors |
| Acute renal failure |
| Age younger than one year |
| Chronic kidney disease stage 4 or 5 (glomerular filtration rate < 30 mL per minute or dialysis) |
| Hepatorenal syndrome |
| Perioperative liver transplantation period |
| Mitigating actions |
| Avoid repeat exposures |
| Consider hemodialysis after exposure in patients on dialysis |
| Use gadoteridol* (Prohance) in high-risk patients |
| Use lowest dose of gadolinium agent possible |
| Weigh pros and cons of gadolinium agents in high-risk patients; completely exhaust noncontrast techniques |
Appropriate Use of Imaging
The American Academy of Family Physicians' (AAFP) position paper on radiology discusses ordering and interpreting radiographs in the outpatient setting, and referring for more extensive imaging when indicated.20 Physicians should apply the principles of judicious imaging, which involves ordering the recommended diagnostic procedures for selected common presenting conditions.20 Appropriateness criteria for various imaging modalities for specific clinical scenarios were developed by the American College of Radiology (ACR). The ACR uses a nine-point scale, with a score of 9 representing a test that is considered most appropriate by expert consensus panels.
CENTRAL NERVOUS SYSTEM IMAGING
Suspected Stroke. Multiple guidelines recommend urgent neuroimaging in patients with suspected stroke.21–24 Noncontrast CT to rule out bleeding receives the highest ACR rating (score = 9); it should be the first step within three hours of symptom onset.21 Within three to 24 hours of symptom onset, a combination of vascular and cerebral imaging is recommended with either head and neck CT angiography or magnetic resonance angiography (score = 8 for both).21 Magnetic resonance angiography is preferred if it can be performed without unreasonable delay and with diffusion-weighted imaging, which is superior to CT for diagnosis of acute ischemic stroke (odds ratio = 25; 95% confidence interval [CI], 8 to 79).25,26 Diffusion-weighted imaging measures net movement of water molecules and predicts patient functional outcomes in anterior-circulation stroke syndromes.25 However, it has not achieved ideal sensitivity (currently 80 to 90 percent) for ischemic stroke and cannot reliably exclude hemorrhage acutely; it cannot be a stand-alone test in the first three hours of symptom onset.25
Headache. Headaches without focal neurologic deficit rarely represent serious pathology, such as tumor or aneurysm; one study showed a 0.4 percent yield of potentially treatable lesions in patients with nontraumatic migraine.27 The presence of focal symptoms or signs increases the likelihood of finding an abnormality on neuroimaging (positive likelihood ratio [LR+] = 3.0; 95% CI, 2.3 to 4.0).28 The ACR rates CT or MRI as likely appropriate in this composite group (score = 7 to 8).27 Special populations that may merit imaging include those with immunosuppression, thunderclap onset, new onset in pregnancy, suspected meningitis, and ipsilateral Horner syndrome associated with neck arterial dissection.27 Chronic nonspecific headaches without new features, such as tension-type headaches, may merit imaging in individual cases (score = 4), but clear evidence is lacking.27,28
CHEST IMAGING
Pulmonary Embolism. Pulmonary embolism presents with dyspnea, chest pain, and tachycardia. If a pretest probability tool, such as the Wells score, suggests intermediate or high risk of pulmonary embolism, then diagnostic imaging with CT is required.29,30 Joint clinical guidelines from the AAFP and the American College of Physicians state that helical CT has a maximal sensitivity of 90 percent and specificity of 95 percent compared with conventional pulmonary arteriography, the traditional standard of care; hence, further studies such as technetium 99m ventilation-perfusion (V/Q) scanning or pulmonary arteriography are indicated in patients with very high probability of pulmonary embolism and a negative CT.29
Given the technologic advancements that have been made since publication of the joint guidelines,29 the ACR rates chest CT angiography as the current standard of care for diagnosing pulmonary embolism (score = 9). A plain film to first exclude other causes of chest pain and dyspnea is recommended.31 If chest radiography is negative and CT angiography is contraindicated or nondiagnostic, then technetium 99m V/Q scanning is suggested (score = 8).31 Traditional pulmonary arteriography with right heart catheterization (score = 5) has the highest radiation level of all available tests; technical factors and observer variability also downgrade its value.31
Chest Pain. Patients with chest pain who are admitted to rule out myocardial infarction need early diagnostic stratification. Myocardial perfusion imaging has the strongest evidence to test for reversible or irreversible ischemic disease; the ACR gives myocardial perfusion imaging a score of 8 for patients with intermediate or high probability of myocardial infarction.32 Sestamibi is often chosen for its lower radiation dose compared with thallium. Coronary angiography is the diagnostic standard; however, despite having a lower radiation dose than myocardial perfusion imaging, it is still a substantial dose, and is more invasive. The ACR gives arteriography a score of 8 for suspected acute coronary syndrome.32
For patients without recurrent pain or with nondiagnostic electrocardiography or negative biomarkers, the American College of Cardiology recommends stress testing (unspecified type) or coronary CT angiography; radiation exposures from myocardial perfusion imaging or coronary CT angiography are comparable.33
Exercise is the preferred stress agent for myocardial perfusion imaging. For patients with low mobility, adenosine receptor agonists are often chosen. Although sensitivity for detecting angiographically significant disease (i.e., 50 percent or greater reduction in the luminal diameter of at least one vessel) is comparable at 64 percent, exercise has higher specificity than adenosine agonists (65 versus 54 percent).34
ABDOMINAL IMAGING
Appendicitis. In patients with right lower quadrant pain and suspected appendicitis, CT of the abdomen and pelvis with contrast media is recommended for adults (score = 8), with ultrasonography being a viable but consistently less accurate option (score = 6).35 A systematic review confirmed the value of contrast media (intravenous, oral, or rectal) with CT (LR+ = 19; negative likelihood ratio [LR–] = 0.06).36,37
In pregnancy and suspected appendicitis, ultrasonography is preferred (score = 8), with noncontrast MRI of the abdomen and pelvis (score = 7) reserved for negative or equivocal ultrasonography results. For children younger than 14 years, right lower quadrant ultrasonography is also endorsed (score = 8; LR+ = 17; LR– = 0.2), and CT is a second-line option (score = 7).35,36 Although ultrasonography is a safer confirmatory test to rule in appendicitis, CT with contrast media is the most sensitive test that excludes the diagnosis (LR– = 0.00 to 0.09).37
Cholecystitis. Minimal confusion exists for evaluation of right upper quadrant pain: ultrasonography is the preferred initial imaging modality for suspected acute cholecystitis or cholelithiasis (score = 8 to 9; LR+ = 2.6; LR– = 0.5).38 For equivocal findings, contrast CT, cholescintigraphy, and contrast MRI have similar levels of supporting evidence (score = 6 for each); however, cholescintigraphy has the best discrimination to evaluate acute cholecystitis (LR+ = 4.2; LR– = 0.04).36,38
Diverticulitis. For adults with acute or chronic left lower quadrant pain with or without fever, contrast CT of the abdomen and pelvis is the preferred modality (score = 8 to 9). Although ultrasonography in women of childbearing age is the suggested first-line modality (score = 4), it is used only to exclude left lower quadrant gynecologic causes, not to evaluate gastrointestinal etiologies.39 Barium enema is no longer considered the preferred method for diverticulitis evaluation (score = 4).39 Plain film has limited value (score = 5).39 MRI is usually not indicated (score = 5).39
Urolithiasis. Patients with suspected acute urolithiasis should undergo noncontrast CT of the abdomen and pelvis (score = 8); traditional intravenous urography is considered to be a second-line modality (score = 4).40 In pregnancy, ultrasonography is used initially (score = 6), with limited three-view intravenous urography if results are negative; physicians should order baseline films of the kidneys, ureters, and bladder, with repeat films 15 and 60 minutes after contrast media administration.41 If patients are allergic to iodinated contrast agents, ultrasonography should be used initially.40
LUMBAR IMAGING
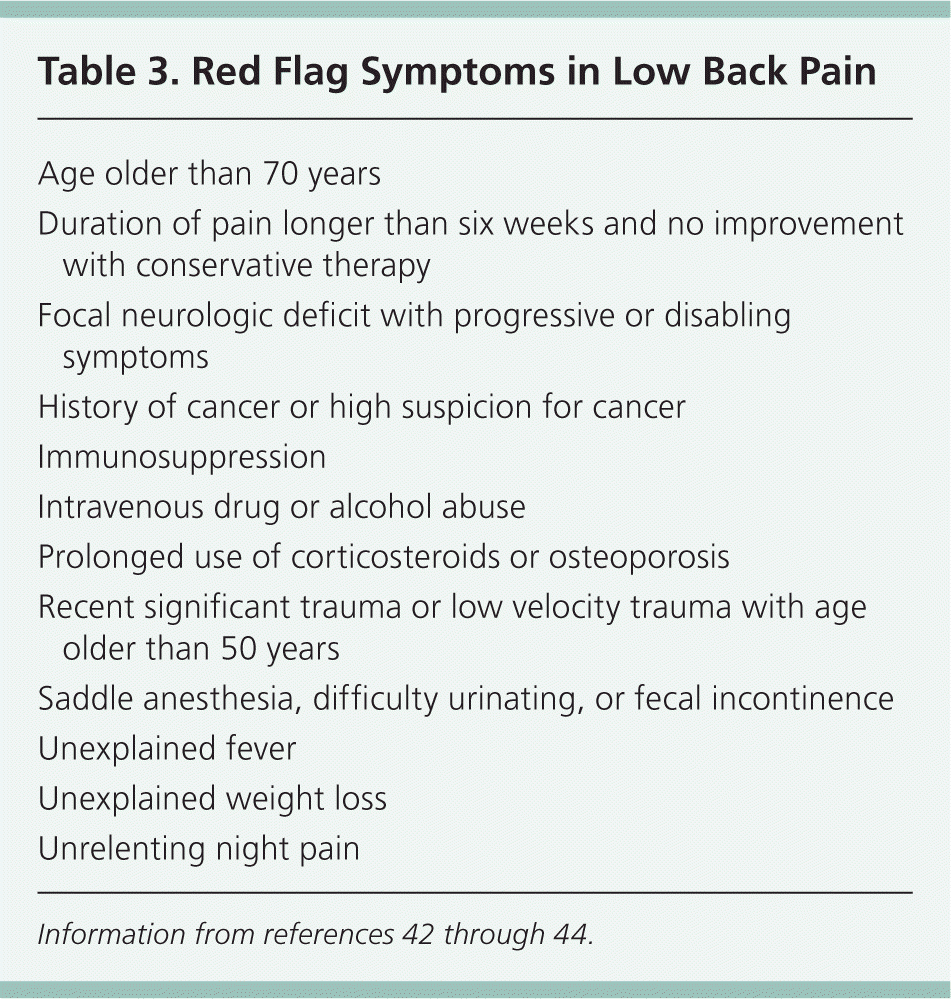
Low Back Pain. In acute nonspecific low back pain, imaging should be avoided unless red flag symptoms are present (Table 342–44 ). Note that radicular pain in the absence of focal neurologic deficit is not a red flag.42 The importance of avoiding unnecessary imaging in nonspecific low back pain is highlighted by its inclusion as a national quality measure (i.e., the percentage of patients with nonspecific low back pain in the absence of red flags who receive imaging studies during the first six weeks of pain symptoms).43
| Age older than 70 years |
| Duration of pain longer than six weeks and no improvement with conservative therapy |
| Focal neurologic deficit with progressive or disabling symptoms |
| History of cancer or high suspicion for cancer |
| Immunosuppression |
| Intravenous drug or alcohol abuse |
| Prolonged use of corticosteroids or osteoporosis |
| Recent significant trauma or low velocity trauma with age older than 50 years |
| Saddle anesthesia, difficulty urinating, or fecal incontinence |
| Unexplained fever |
| Unexplained weight loss |
| Unrelenting night pain |
If films are indicated, oblique view radiography is not recommended. It rarely changes the working diagnosis, and increases radiation exposure more than twofold.42 The ACR rates all imaging modalities inappropriate for acute nonspecific low back pain (score = 2).42 Although 90 percent of primary care patients do not seek additional medical care after three months, only 25 percent are completely symptom-free 12 months later.45
In patients with low back pain lasting more than six weeks, MRI may be considered for suspected spinal malignancy, infection, fracture, or cauda equina syndrome, plus those with a history of lumbar surgery, osteoporosis, immunosuppression, ankylosing spondylitis, or other inflammatory disorders. MRI should not be performed for nonspecific chronic low back pain.42,46 Rationale for minimizing MRI use is the high rate of abnormal findings even in asymptomatic patients. In one study, 64 percent of persons without back pain had an intervertebral disk abnormality on MRI, and 38 percent had abnormalities at multiple levels.47 The primary exception is the presence of severe nonspecific pain when all nonsurgical treatment options have been ineffective and the patient desires spinal fusion.46 Bone scintigraphy with single-photon emission CT is useful when spondylolysis is suspected based on physical examination or suggestive plain films.42
WHOLE BODY SCANNING
Whole body scanning has been marketed for self-referral and detection of preclinical disease. Most results (up to 80 percent in older patients) will have at least one abnormality; the mean is three findings per patient.48 Despite most findings being benign, approximately one-third of patients are referred for follow-up imaging.48 Most major medical organizations recommend against whole body scanning, and none endorse it as a screening tool in unselected populations (Table 4).48–51

| Risks |
| Detection of incidentalomas* |
| High false-negative rate |
| High false-positive rate (e.g., lung parenchymal scars, granulomas, indeterminate nodules, emphysema) |
| Increased rate of overdiagnosis |
| Overexposure to radiation (500 to 1,000 times higher than routine chest radiography; e.g., abdominal/pelvic vascular calcifications, hepatorenal cysts, urolithiasis, nonspecific lesions) |
| Physical damage caused by objects moving in soft tissue during magnetic imaging |
| Unnecessary examinations/biopsy |
| Unnecessary exposure to intravenous contrast |
| Unnecessary patient anxiety |
| Organizations recommending against whole body scanning |
| American Association of Physicists in Medicine |
| American College of Cardiology/American Heart Association |
| American College of Radiology |
| American Medical Association |
| Health Physics Society |
| U.S. Food and Drug Administration |
| U.S. Preventive Services Task Force |
Data Sources: The following evidence-based medicine resources were searched using the key words diagnostic imaging, radiation risk, ionizing radiation, whole body scan, nephrogenic systemic fibrosis, diffusion-weighted imaging, headache, chest pain, abdominal pain, suspected stroke, and back pain: Essential Evidence Plus, the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, Database of Abstracts of Reviews of Effects, the Institute for Clinical Systems Improvement, the National Guideline Clearinghouse database, PubMed, the Trip database, and UpToDate. Search date: October 1, 2010.
