
Am Fam Physician. 2013;87(10):699-705
Author disclosure: No relevant financial affiliations to disclose.
Ulcerative colitis is a chronic inflammatory disease of the colon. The etiology is unknown. Risk factors include a history of recent infection with Salmonella or Campylobacter, living in Western industrialized nations and at higher latitudes, and a family history of the disease. The incidence peaks in early adulthood, but patients can develop the disorder from early childhood through adulthood. Ulcerative colitis often presents with abdominal pain, diarrhea, and hematochezia. It is important to exclude infectious etiologies. Anemia and an elevated erythrocyte sedimentation rate or C-reactive protein level may suggest inflammatory bowel disease, but the absence of laboratory abnormalities does not rule out ulcerative colitis. The diagnosis is suspected clinically and confirmed through endoscopic biopsy. First-line treatment is therapy with 5-aminosalicylic acid. Corticosteroids may be added if 5-aminosalicylic acid therapy is ineffective. Infliximab can be added to induce and sustain remission. Patients with severe or nonresponsive ulcerative colitis should be hospitalized, and intravenous corticosteroids should be given. If medical management has been ineffective, surgical intervention is indicated for severe disease. Patients with ulcerative colitis have an increased risk of colon cancer and should have periodic colonoscopy beginning eight to 10 years after diagnosis.
Ulcerative colitis is a disease of the colon characterized by chronic inflammation. The cause of the aberrant immune response is unclear, but genetic, dietary, and environmental risk factors have a role. In contrast with that of Crohn's disease, the inflammation of ulcerative colitis is limited to the colonic mucosa. The portion of the colon affected varies. Some patients have inflammation that is limited to the rectum (ulcerative proctitis), whereas others have more proximal disease. Pancolitis refers to ulcerative colitis that affects the entire colon.1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| 5-Aminosalicylic acid is highly effective for inducing remission and preventing relapse in patients with ulcerative colitis. | A | 21 |
| Oral corticosteroids are effective for inducing remission in patients with ulcerative colitis. | B | 23 |
| Infliximab (Remicade) is effective for inducing remission in patients with corticosteroid-refractory ulcerative colitis. | A | 24 |
| Azathioprine (Imuran) is effective for preventing relapse in patients with ulcerative colitis. | B | 25 |
| The probiotics Lactobacillus GG and Escherichia coli Nissle 1917 (Mutaflor) are as effective as 5-aminosalicylic acid in maintaining remission in patients with ulcerative colitis. | B | 16, 17 |
| Colonoscopy should be initiated eight to 10 years after ulcerative colitis is diagnosed, with regular-interval biopsies every one to two years. | C | 14 |
Demographics
The annual incidence of ulcerative colitis in the United States is between nine and 12 cases per 100,000 persons.1 Inflammatory bowel disease is more common in industrialized countries and in Western nations. The incidence is also increased in persons who live at higher latitudes.1 Worldwide and in less developed nations, ulcerative colitis is more common than Crohn's disease, whereas in some studies of U.S. populations, the incidence of the two disorders is almost equal.1 Four percent of cases of inflammatory bowel disease cannot be characterized definitively as either Crohn's disease or ulcerative colitis; these patients are said to have indeterminate colitis.2
The incidence of ulcerative colitis is similar between men and women, in contrast with Crohn's disease, which has a higher incidence in women. In the United States, the incidence of ulcerative colitis does not vary significantly by race. Smokers and persons who have had an appendectomy are less likely to develop ulcerative colitis.1 Although ulcerative colitis can occur from infancy on, the risk climbs dramatically in early adulthood and trends slightly downward from that point on.2 In the past, inflammatory bowel disease was described as having a bimodal distribution, with peaks of incidence in the late teens to early 20s and again in older age. More recent studies have not shown this pattern.3
Infection with nontyphoid Salmonella or Campylobacter is associated with an eight to 10 times higher risk of developing ulcerative colitis in the following year.4 The risk diminishes with time, but is still present up to 10 years later. The intestinal bacterial flora of patients with inflammatory bowel disease has been shown to be markedly abnormal, but this finding has not yet led to therapeutic interventions.5
Genetic factors have a role in ulcerative colitis. Having a sibling with ulcerative colitis increases the risk of developing the disease 4.6-fold, and the relative risk of one monozygotic twin having the disease is 95 times higher if the other twin is affected.6 Dietary factors also affect the risk of ulcerative colitis. A diet high in refined sugar, fat, and meat increases the risk, whereas a diet rich in vegetables reduces the risk.1,7 The hygiene hypothesis states that excessive hygiene habits in Western industrialized nations prevent children from normal exposure to bacterial and helminthic antigens, thereby changing immune system responsiveness. Epidemiologic data provide indirect evidence for this theory, in the same way that they do for asthma and allergies.1
Diagnosis
Ulcerative colitis typically presents with hematochezia, diarrhea, and abdominal pain. The onset of symptoms can be sudden or gradual.8 The presence of anemia, thrombocytosis, or hypoalbuminemia may suggest inflammatory bowel disease, but most patients with ulcerative colitis will not have these abnormalities.9 C-reactive protein level and erythrocyte sedimentation rate are relatively insensitive for detecting ulcerative colitis and should not be relied on to exclude inflammatory bowel disease. At the time of diagnosis, fewer than one-half of patients with ulcerative colitis have abnormal findings on these tests.9,10 Endoscopic biopsy is used to confirm the diagnosis.11
Elevated fecal calprotectin and lactoferrin levels have been proven sensitive for the detection of inflammatory bowel disease, but using these tests to exclude patients from endoscopic examination delays diagnosis in 6 to 8 percent of patients.10 Tests for perinuclear antineutrophil cytoplasmic antibodies and anti– Saccharomyces cerevisiae antibodies are often positive in patients with ulcerative colitis, and can help distinguish the condition from Crohn's disease in those with indeterminate histology.10 Approximately one-third of patients with ulcerative colitis have extraintestinal manifestations, which may be present even when the disease is inactive (Table 1).12
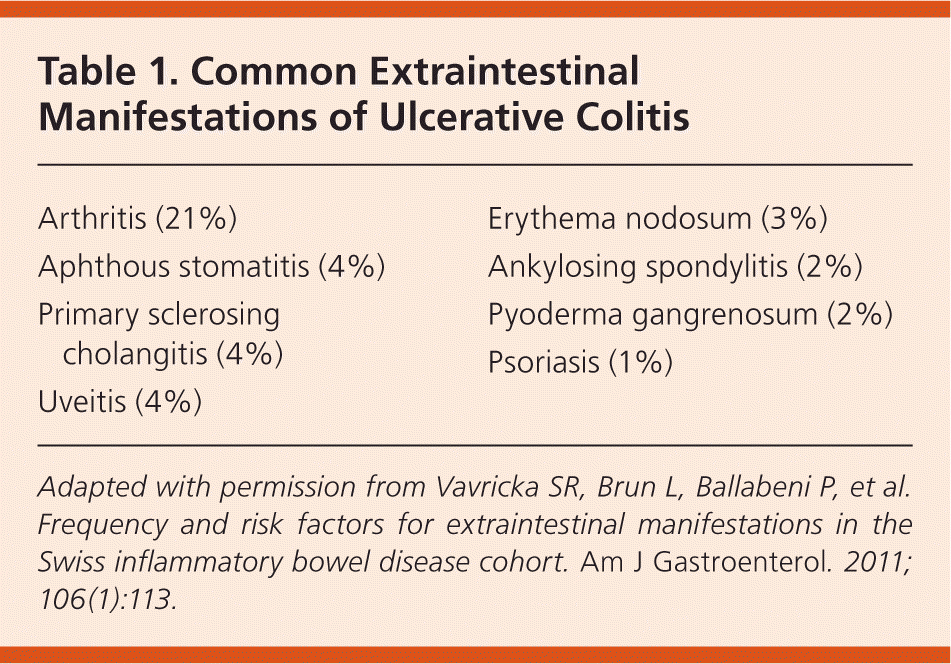
| Arthritis (21%) |
| Aphthous stomatitis (4%) |
| Primary sclerosing cholangitis (4%) |
| Uveitis (4%) |
| Erythema nodosum (3%) |
| Ankylosing spondylitis (2%) |
| Pyoderma gangrenosum (2%) |
| Psoriasis (1%) |
Differential Diagnosis
The differential diagnosis for ulcerative colitis includes Crohn's disease and infectious colitis caused by bacterial, viral, or parasitic pathogens. Ulcerative colitis usually presents with contiguous disease, whereas patients with Crohn's disease may have areas of normal mucosa between areas of disease. Ulcerative colitis must also be distinguished from microscopic colitis, a common cause of nonbloody diarrhea, abdominal pain, and weight loss in adults. Microscopic colitis is diagnosed with endoscopic biopsy.13 Patients in whom ulcerative colitis is suspected should have bacterial stool cultures performed. Those with a history of recent antibiotic use should be tested for Clostridium difficile toxin. Additional testing to exclude infectious etiologies may be performed depending on the patient's history 14 (Table 214,15 ).
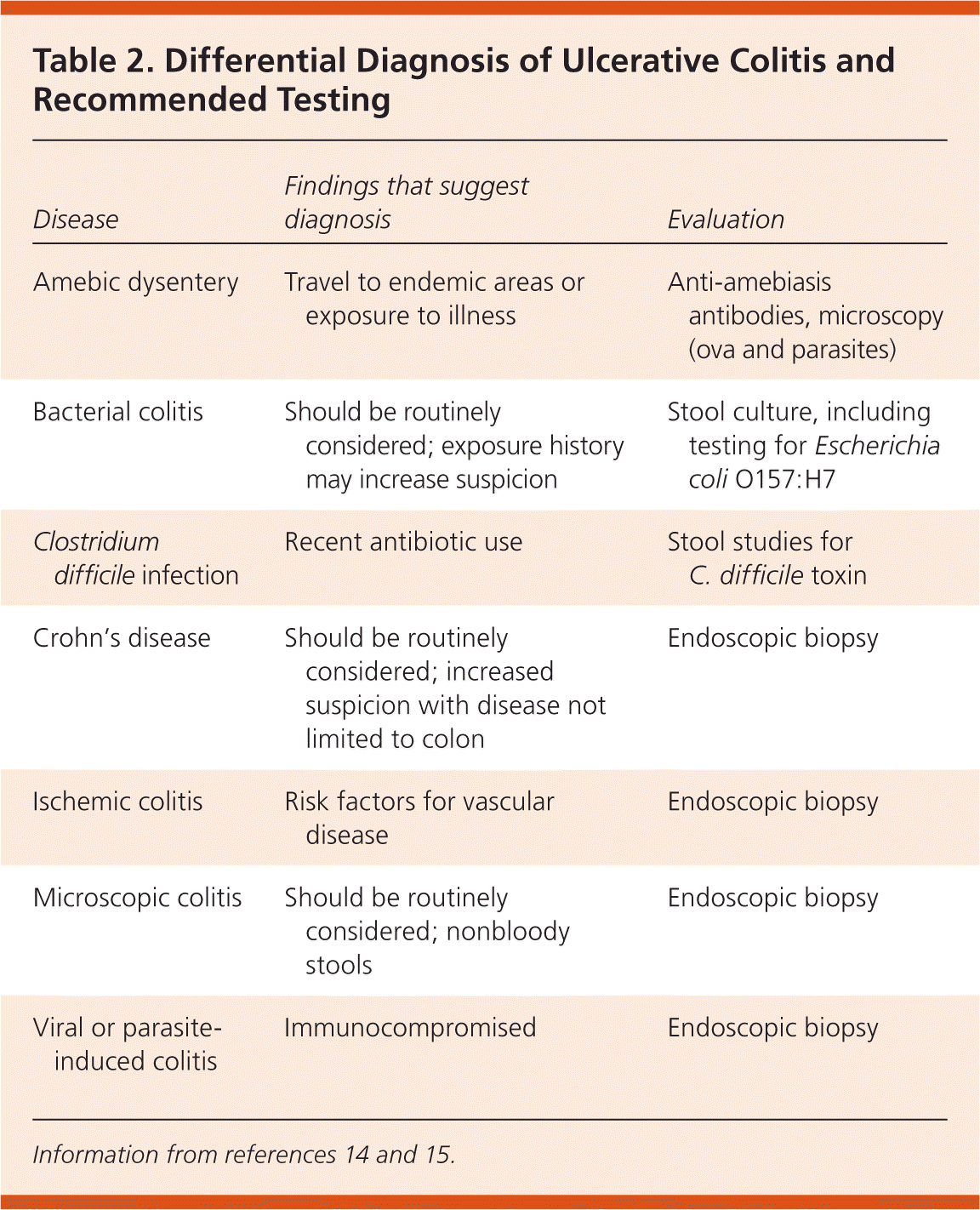
| Disease | Findings that suggest diagnosis | Evaluation |
|---|---|---|
| Amebic dysentery | Travel to endemic areas or exposure to illness | Anti-amebiasis antibodies, microscopy (ova and parasites) |
| Bacterial colitis | Should be routinely considered; exposure history may increase suspicion | Stool culture, including testing for Escherichia coli O157:H7 |
| Clostridium difficile infection | Recent antibiotic use | Stool studies for C. difficile toxin |
| Crohn's disease | Should be routinely considered; increased suspicion with disease not limited to colon | Endoscopic biopsy |
| Ischemic colitis | Risk factors for vascular disease | Endoscopic biopsy |
| Microscopic colitis | Should be routinely considered; nonbloody stools | Endoscopic biopsy |
| Viral or parasite-induced colitis | Immunocompromised | Endoscopic biopsy |
Treatment
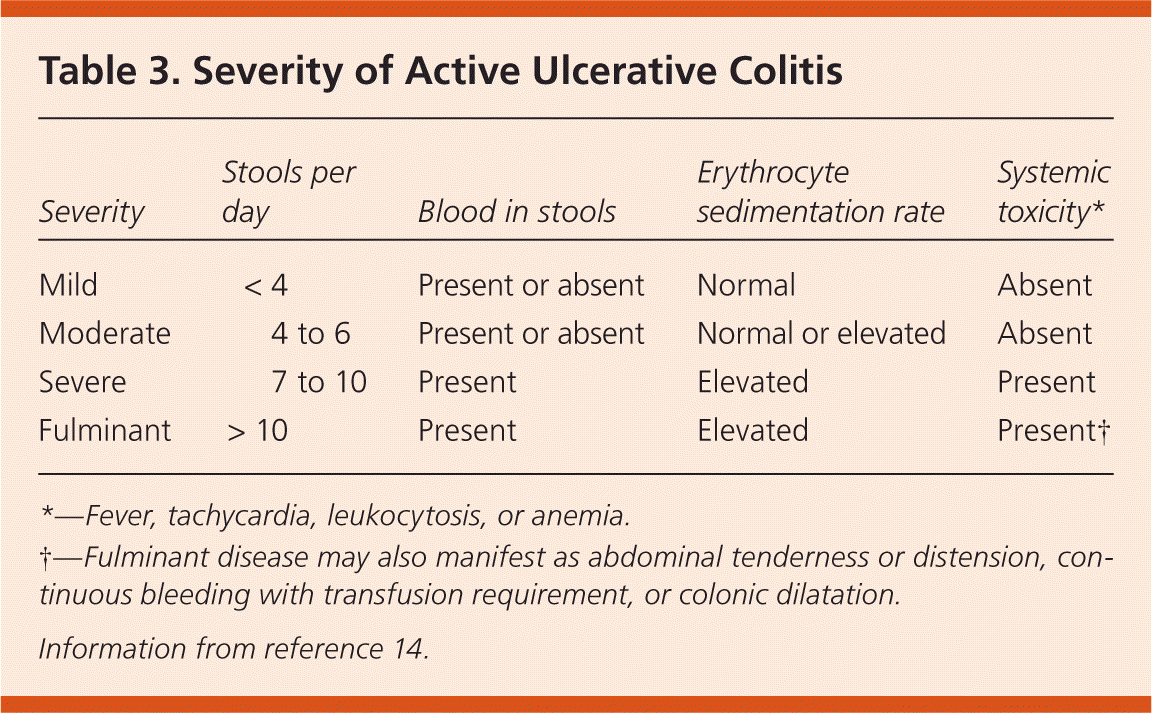
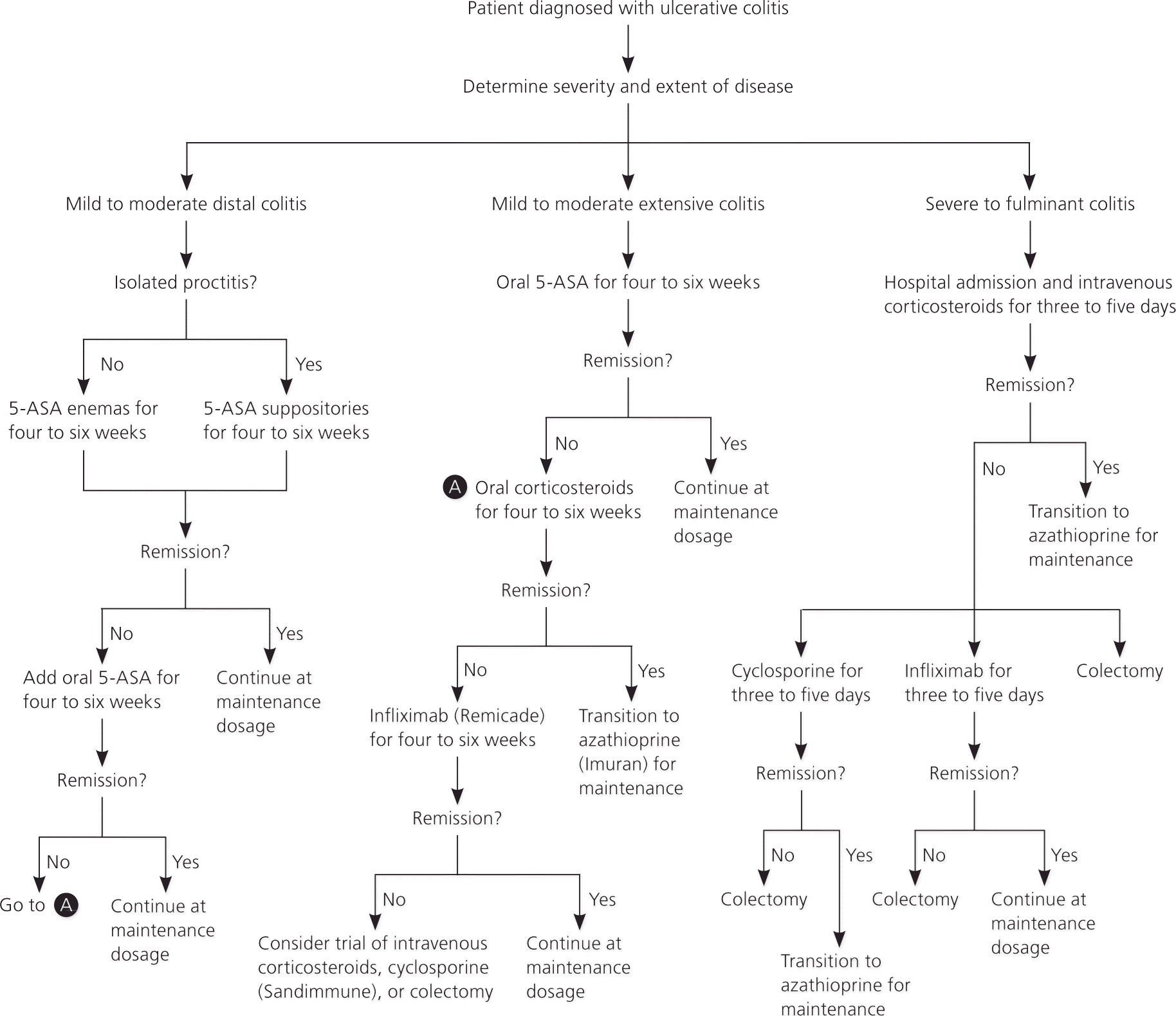
The goals of treatment in ulcerative colitis are to induce remission of active disease and prevent relapse. The preferred method of treatment for active disease is determined by the endoscopic extent and clinical severity of disease (Table 3).14 The same agent used for remission is generally used for maintenance, but at a lower dosage (Table 4).14,16,17 A treatment approach is presented in Figure 1.14
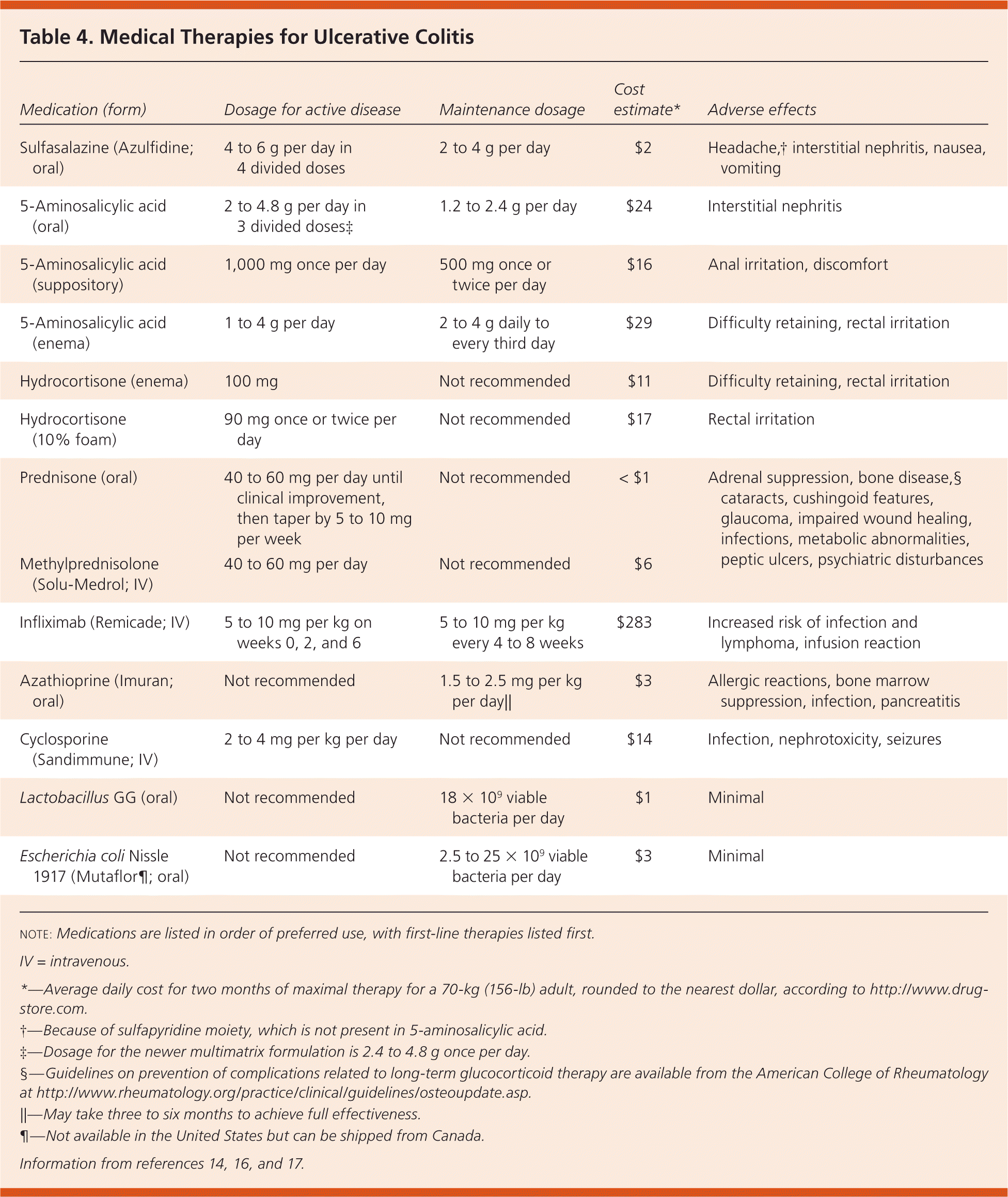
| Medication (form) | Dosage for active disease | Maintenance dosage | Cost estimate* | Adverse effects |
|---|---|---|---|---|
| Sulfasalazine (Azulfidine; oral) | 4 to 6 g per day in 4 divided doses | 2 to 4 g per day | $2 | Headache,† interstitial nephritis, nausea, vomiting |
| 5-Aminosalicylic acid (oral) | 2 to 4.8 g per day in 3 divided doses‡ | 1.2 to 2.4 g per day | $24 | Interstitial nephritis |
| 5-Aminosalicylic acid (suppository) | 1,000 mg once per day | 500 mg once or twice per day | $16 | Anal irritation, discomfort |
| 5-Aminosalicylic acid (enema) | 1 to 4 g per day | 2 to 4 g daily to every third day | $29 | Difficulty retaining, rectal irritation |
| Hydrocortisone (enema) | 100 mg | Not recommended | $11 | Difficulty retaining, rectal irritation |
| Hydrocortisone (10% foam) | 90 mg once or twice per day | Not recommended | $17 | Rectal irritation |
| Prednisone (oral) | 40 to 60 mg per day until clinical improvement, then taper by 5 to 10 mg per week | Not recommended | < $1 | Adrenal suppression, bone disease,§ cataracts, cushingoid features, glaucoma, impaired wound healing, infections, metabolic abnormalities, peptic ulcers, psychiatric disturbances |
| Methylprednisolone (Solu-Medrol; IV) | 40 to 60 mg per day | Not recommended | $6 | |
| Infliximab (Remicade; IV) | 5 to 10 mg per kg on weeks 0, 2, and 6 | 5 to 10 mg per kg every 4 to 8 weeks | $283 | Increased risk of infection and lymphoma, infusion reaction |
| Azathioprine (Imuran; oral) | Not recommended | 1.5 to 2.5 mg per kg per day|| | $3 | Allergic reactions, bone marrow suppression, infection, pancreatitis |
| Cyclosporine (Sandimmune; IV) | 2 to 4 mg per kg per day | Not recommended | $14 | Infection, nephrotoxicity, seizures |
| Lactobacillus GG (oral) | Not recommended | 18 × 109 viable bacteria per day | $1 | Minimal |
| Escherichia coli Nissle 1917 (Mutaflor¶; oral) | Not recommended | 2.5 to 25 × 109 viable bacteria per day | $3 | Minimal |
ACTIVE DISEASE
For active disease distal to the descending colon, topical 5-aminosalicylic acid (5-ASA), including suppository and enema formulations, is the preferred treatment.18 Topical 5-ASA is more effective than oral 5-ASA or topical corticosteroid foams or enemas. Suppositories are effective for proctitis, whereas enemas are effective for disease that reaches as proximally as the splenic flexure.18 Topical mesalamine enemas, a common formulation of 5-ASA, induce remission in 72 percent of cases of active, left-sided ulcerative colitis within four weeks.19 Corticosteroid foams are also effective, although less so than 5-ASA. However, they may be easier to administer and more comfortable to retain.18 Oral 5-ASA is effective and may be preferred by patients who have difficulty with irritation or retaining topical formulations.18 A combination of oral and topical 5-ASA is superior to either formulation alone and should be used when other treatments are ineffective.20
Oral 5-ASA is effective for mild to moderate active ulcerative colitis extending proximal to the sigmoid colon.21 If oral 5-ASA is ineffective, the addition of topical 5-ASA can be more effective than oral treatment alone.22 A short-term course of oral corticosteroids may be effective if the disease does not respond to combination 5-ASA therapy, or for patients in whom a more rapid response is desired.23 Infliximab (Remicade), an intravenously administered monoclonal antibody to tumor necrosis factor-α, is effective for corticosteroid-refractory disease.24 A recent meta-analysis of studies of azathioprine (Imuran) for treatment of active ulcerative colitis showed no statistically significant effect.25
Patients with severe to fulminant disease can be divided into those who require urgent hospitalization and those who may receive a trial of outpatient treatment. Signs and symptoms that suggest the need for urgent hospitalization include severe pain, abdominal or colonic distension, gastrointestinal bleeding, and toxicity (e.g., fever, tachycardia, leukocytosis, anemia). In patients who do not require urgent hospitalization, a trial of maximal dosage combined oral/topical 5-ASA therapy is recommended in conjunction with oral corticosteroids. If symptoms do not improve and the patient still does not require urgent hospitalization, the addition of infliximab as outpatient therapy is another option.14
For patients who do not improve on maximal outpatient medical management or who have signs of toxicity, hospital admission and administration of intravenous corticosteroids reduce morbidity and mortality.26 If intravenous corticosteroids are ineffective after three to five days, intravenous cyclosporine (Sandimmune) or infliximab increases remission rates.24,27
For patients who do not improve with the addition of cyclosporine or infliximab, there is evidence that switching to the other agent may decrease the risk of colectomy; however, this is associated with an increased risk of infectious complications, and the decision should be individualized.27 For patients with severe acute or chronic colitis who do not improve with medical therapy, surgical proctocolectomy is the next step. Proctocolectomy with ileal pouch-anal anastomosis is most common; however, permanent ileostomy is also an option. Other indications for surgery are exsanguinating hemorrhage, perforation, or carcinoma.14 A recent systematic literature review showed that 12 months after surgery, health-related quality of life and health status are equivalent to that in the general population.28 Despite the potential benefits of surgery, complications are a concern. Colectomy is associated with a 54 percent reoperation rate for postsurgical complications.29 Pouchitis (inflammatory disease of the ileal pouchanal anastomosis pouch of unknown etiology) is also common.14,29
MAINTENANCE
Once remission is induced, the same agent is usually used to maintain remission. 5-ASA suppositories and enemas are effective for maintenance of distal disease.30,31 Oral 5-ASA is effective for extensive colitis.21 As with active disease, combined oral/topical therapy is more effective in maintaining remission than either agent alone.32 Corticosteroids are not effective in maintaining remission and have potentially serious adverse effects with long-term use.33 They should not be used for maintenance therapy. Azathioprine is an option for patients who require corticosteroids or cyclosporine for induction of remission or in whom remission is not adequately maintained with 5-ASA. However, it usually takes several months before it reaches full effect.25 For patients in whom remission was induced using infliximab, the same agent may be continued as maintenance therapy.24
COMPLEMENTARY AND ALTERNATIVE MEDICINE
Probiotics may modestly reduce disease activity in active disease, but do not increase remission rates.34 However, Lactobacillus GG and Escherichia coli Nissle 1917 (Mutaflor) have been shown to be as effective as 5-ASA for maintenance therapy.16,17 Acupuncture has shown a small symptomatic benefit in active disease.35 Wheatgrass has also shown some effectiveness in reducing symptoms of active disease.36
Monitoring
In addition to disease-specific considerations, patients with ulcerative colitis have the same requirements for preventive health care as the general population.
VACCINATIONS
There are no specific vaccination recommendations for patients with ulcerative colitis. However, if systemic immunosuppressive therapies (e.g., 5-ASA, corticosteroids, infliximab, cyclosporine, azathioprine) are used, patients should receive pneumococcal and influenza vaccinations. Before beginning systemic immunosuppressive therapy, other vaccinations should be reviewed and updated, if needed. Live vaccines are contraindicated, and inactivated vaccines may elicit a suboptimal response in patients receiving systemic immunosuppressive therapy.37
SCREENING
Patients with ulcerative colitis have special considerations for routine screening examinations. These patients have increased rates of abnormal Papanicolaou test results, but are screened at a suboptimal rate.38 The American College of Obstetricians and Gynecologists recommends more frequent screening in patients taking immunosuppressive medications, but it does not define the screening interval. Yearly screening is a reasonable option.39
Increased rates of osteoporosis are another concern in persons with ulcerative colitis. Dual energy x-ray absorptiometry should be performed in patients with risk factors (e.g., smokers, low body mass index, family history) who are older than 60 years or who are on frequent or prolonged corticosteroid therapy.14
Patients with ulcerative colitis have an increased risk of colorectal cancer. Although there is no evidence that cancer screening prolongs survival, screening detects cancers earlier, when they are more treatable.40 The American College of Gastroenterology recommends screening with colonoscopy eight to 10 years after colitis is diagnosed.14 Additionally, random biopsies spaced at regular intervals throughout the colon are recommended every one to two years during screening colonoscopies. Patients with proctitis or proctosigmoiditis do not seem to be at increased risk of cancer.14
Data Sources: A Medline search was performed using the key term ulcerative colitis. It included meta-analyses, randomized controlled trials, clinical trials, and systematic reviews. Also searched were Essential Evidence Plus, the National Guideline Clearinghouse, the U.S. Preventive Services Task Force, and UpToDate. Search period: March to November 2011.
