
Am Fam Physician. 2013;87(11):766-772
A more recent article on pharmacologic therapy for acute pain is available.
Author disclosure: No relevant financial affiliations.
The approach to patients with acute pain begins by identifying the underlying cause and a disease-specific treatment. The first-line pharmacologic agent for the symptomatic treatment of mild to moderate pain is acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID). The choice between these two medications depends on the type of pain and patient risk factors for NSAID-related adverse effects (e.g., gastrointestinal, renovascular, or cardiovascular effects). Different NSAIDs have similar analgesic effects. However, cyclooxygenase-2 selective NSAIDs (e.g., celecoxib) must be used with caution in patients with cardiovascular risk factors and are more expensive than nonselective NSAIDs. If these first-line agents are not sufficient for mild to moderate pain, medications that target separate pathways simultaneously, such as an acetaminophen/opioid combination, are reasonable choices. Severe acute pain is typically treated with potent opioids. At each step, adjuvant medications directed at the underlying condition can be used. Newer medications with dual actions (e.g., tapentadol) are also an option. There is little evidence that one opioid is superior for pain control, but there are some pharmacologic differences among opioids. Because of the growing misuse and diversion of controlled substances, caution should be used when prescribing opioids, even for short-term treatment. Patients should be advised to properly dispose of unused medications.
Acute and chronic pain have been increasingly recognized as being on a continuum, with their development influenced by the initial pain experience and individual biopsychosocial factors.1 A survey involving adults showed that during a three-month period, 29% experienced low back pain, 17% experienced a migraine or severe headache, 15% experienced neck pain, and 5% experienced facial or jaw pain.2 In a survey of ambulatory office visits, analgesics were the most commonly continued or newly prescribed medication at a rate of 11.4%.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Acetaminophen is the first-line treatment for most mild to moderate acute pain. | A | 8, 18 |
| Ibuprofen and naproxen (Naprosyn) are good, first-line NSAIDs for mild to moderate acute pain based on effectiveness, adverse effect profile, cost, and over-the-counter availability. | A | 12, 13 |
| Cyclooxygenase-2 selective NSAIDs are second-line medications for mild to moderate pain based on their similar effectiveness to nonselective NSAIDs and greater costs. | A | 13 |
| Celecoxib (Celebrex) alone and an NSAID plus a proton pump inhibitor have the same probability of causing gastrointestinal complications in those at high risk. | B | 26, 27 |
| Full opioid agonists may be used if opioids combined with acetaminophen or NSAIDs are insufficient to control moderate to severe pain. | A | 14, 15, 31 |
| Tramadol (Ultram) is less effective than hydrocodone/acetaminophen and is a second-line medication for the treatment of moderate to severe pain. | B | 16, 39 |
Approach to the Patient
A focused clinical assessment, based on body region, can help determine the cause of pain.4 During the assessment for underlying conditions, acute pain can be controlled using short-term pharmacologic treatment (with or without nonpharmacologic treatments). Regular evaluation of pain control using a pain scale allows the physician to monitor treatment effectiveness and to determine when changes are warranted.1 Scheduled, rather than as-needed dosing, provides more consistent drug levels and therefore more consistent pain control.
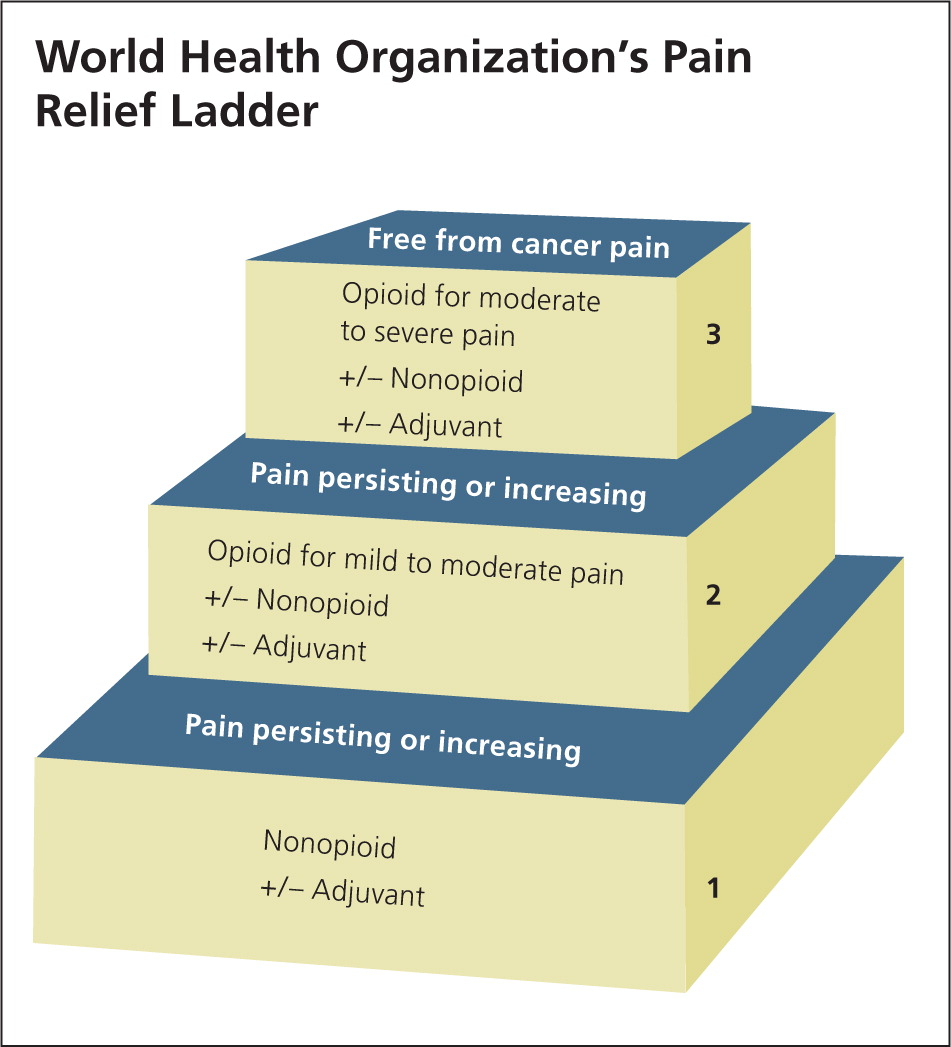
The World Health Organization's (WHO's) pain relief ladder (Figure 1) provides a stepped approach to the management of cancer pain and can also be used for patients with acute and chronic nonmalignant pain.5 Adjuvant medications can be initiated as needed at any step of the ladder.1 These medications include antidepressants (e.g., tricyclics for acute neuropathic pain), anticonvulsants (e.g., gabapentin [Neurontin]), and glucocorticoids (e.g., dexamethasone to reduce postoperative pain, nausea, and vomiting). The analgesic effectiveness increases with each step up on the ladder, as does the potential for medication abuse or addiction. Table 1 summarizes medications used to treat acute pain in adults.6–16
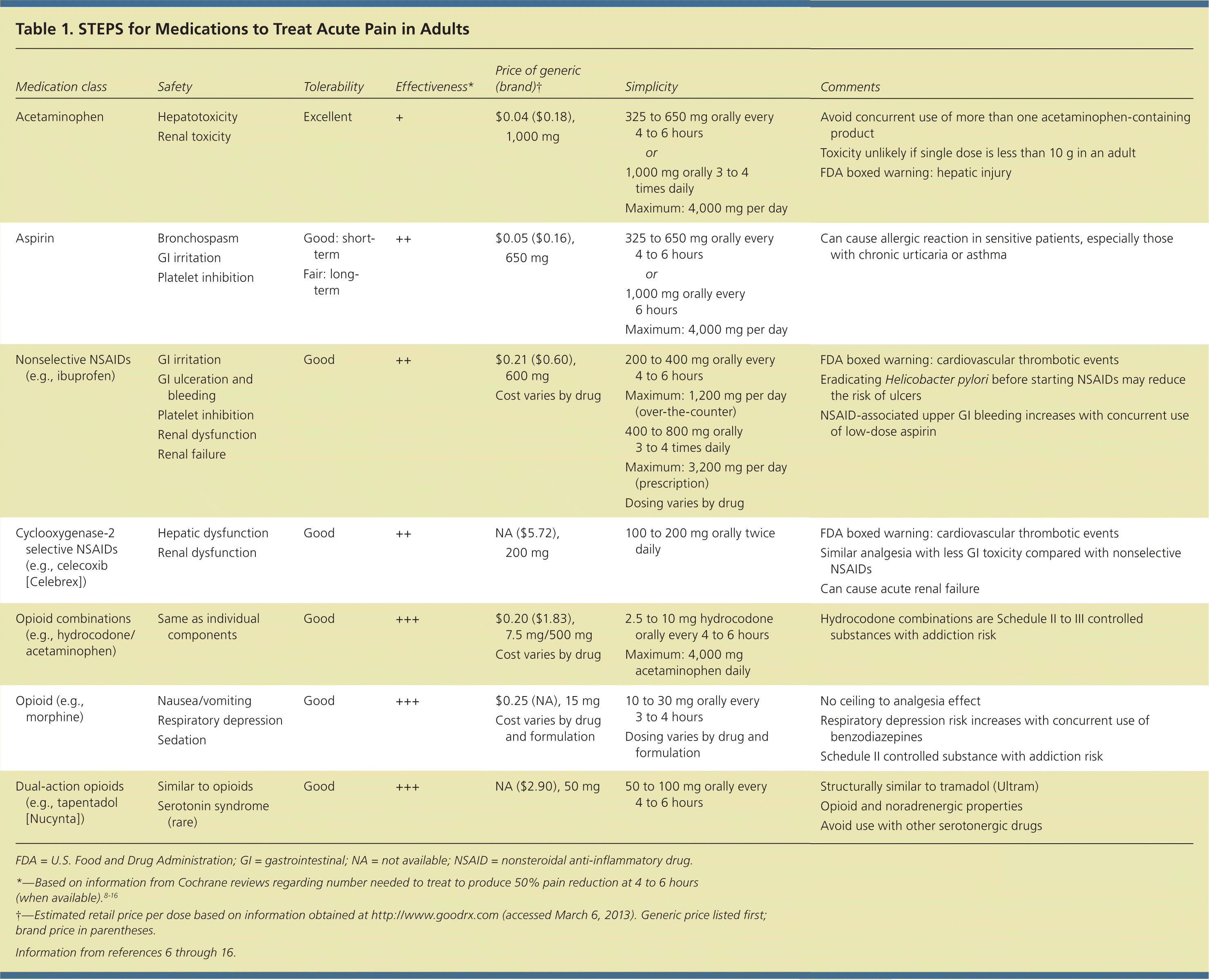
| Medication class | Safety | Tolerability | Effectiveness* | Price of generic (brand)† | Simplicity | Comments | |
|---|---|---|---|---|---|---|---|
| Acetaminophen |
|
| + |
|
|
| |
| Aspirin |
|
| ++ |
|
|
| |
| Nonselective NSAIDs (e.g., ibuprofen) |
|
| ++ |
|
|
| |
| Cyclooxygenase-2 selective NSAIDs (e.g., celecoxib [Celebrex]) |
|
| ++ |
|
|
| |
| Opioid combinations (e.g., hydrocodone/acetaminophen) |
|
| +++ |
|
|
| |
| Opioid (e.g., morphine) |
|
| +++ |
|
|
| |
| Dual-action opioids (e.g., tapentadol [Nucynta]) |
|
| +++ |
|
|
| |
Initial Pharmacotherapy
The WHO pain relief ladder recommends a nonopioid such as acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) for the initial management of pain. Acute pain characteristics and patient risk factors should be considered when choosing between acetaminophen and an NSAID (e.g., aspirin, other nonselective NSAIDs, cyclooxygenase-2 [COX-2] selective NSAIDs).
ACETAMINOPHEN
Acetaminophen, called paracetamol outside of the United States, is the first-line treatment for most mild to moderate acute pain.8 The effectiveness of acetaminophen is similar to that of NSAIDs such as celecoxib (Celebrex), 200 mg; aspirin, 600 to 650 mg; and naproxen (Naprosyn), 200 to 220 mg.8 It is generally well-tolerated, has few drug-drug interactions, is not associated with increased blood pressure (as with NSAIDs), can be used during pregnancy (U.S. Food and Drug Administration [FDA] pregnancy category B), and is the analgesic of choice for episodic use in patients with impaired renal function.17 Although acetaminophen is less effective for acute low back pain than some NSAIDs, it is a reasonable first-line option because of its favorable safety and cost profiles.18
ASPIRIN
Aspirin effectively relieves mild to moderate acute pain. It is similar to the same dose of acetaminophen and is comparable to celecoxib, 200 mg.9 Over a dose range of 500 to 1,200 mg, aspirin exhibits a dose-response relationship (i.e., a 1,200-mg dose of aspirin provides better pain relief than 600- to 650-mg doses).9 Like NSAIDs, aspirin can cause gastrointestinal hemorrhage and ulcer.19 Patients with chronic urticaria and asthma have a greater likelihood of salicylate hypersensitivity, which can manifest as bronchospasm (20% and 4%, respectively, compared with 1% in the general population).6
OTHER NONSELECTIVE NSAIDS
Nonselective NSAIDs inhibit both COX-1 and COX-2, whereas COX-2 selective NSAIDs have greater COX-2 selectivity. Inhibition of COX-2 is thought to mediate the analgesic properties of NSAIDs, whereas inhibition of COX-1 appears to be associated with gastrointestinal adverse effects. NSAIDs possess anti-inflammatory effects that are lacking with acetaminophen, and they can be especially useful for the treatment of acute pain associated with prostaglandin-mediated activity, such as dysmenorrhea or osteoarthritis.11,12
Because most NSAIDs have nearly identical analgesic effects, the choice is based on cost, dosing schedule, and the frequency or severity of adverse effects. Some NSAIDs (e.g., indomethacin [Indocin], mefenamic acid [Ponstel]) are now rarely used because of adverse effects. Ibuprofen and naproxen are among the most commonly used NSAIDs in the United States because of their effectiveness, adverse effect profile, cost, and over-the-counter availability.12,13
There is a ceiling to the analgesic effects of NSAIDs but not to their anti-inflammatory effects, although adverse effects may limit upward dosing titration. NSAIDs are more effective than placebo or acetaminophen for primary dysmenorrhea, but they are associated with a higher incidence of adverse effects such as headache, drowsiness, nausea, and indigestion.12 In general, there are no differences among NSAIDs in terms of effectiveness or adverse effects.
For osteoarthritis, NSAIDs provide significantly better pain relief than acetaminophen, but with more gastrointestinal adverse events.11 Some evidence suggests that NSAIDs and acetaminophen may be comparable for mild osteoarthritis pain, whereas NSAIDs may be better for moderate to severe osteoarthritis pain.11
Acetaminophen and NSAIDs are equally effective for acute low back pain, although NSAIDs are associated with a higher incidence of adverse effects.13,18 There is no difference in effectiveness among NSAIDs, narcotic analgesics, and muscle relaxants for acute low back pain.13 Adding a muscle relaxant to an NSAID regimen does not provide further relief for acute low back pain and is associated with more adverse effects.13
Topical NSAIDs are more effective than placebo for treating acute pain (e.g., from strains, sprains, contusions, or overuse injuries) in superficial locations, and the incidence of local and systemic adverse events is similar to placebo.20 Based on the number needed to treat, topical indomethacin is not as effective as topical diclofenac (Solaraze), ibuprofen, ketoprofen, or piroxicam (not available in the United States), which are similarly effective.20
COX-2 Selective NSAIDs. Celecoxib is the only COX-2 selective NSAID still available in the United States, where it is approved for bone or dental pain, dysmenorrhea, headache, osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. Meloxicam (Mobic) is sometimes referred to as a COX-2 selective NSAID but is classified as a nonselective NSAID. COX-2 selective NSAIDs are considered second-line medications for mild to moderate pain because they have similar effectiveness to nonselective NSAIDs but with a greater cost.13 COX-2 selective NSAIDs and traditional NSAIDs are similarly effective for acute low back pain, but COX-2 selective NSAIDs have fewer adverse effects.13
NSAID Complications. NSAIDs should be used cautiously in several patient populations. Risk factors for gastrointestinal bleeding and peptic ulcer disease associated with NSAID use include a history of gastrointestinal bleeding, peptic ulcer, older age, smoking or alcohol use, and longer duration of NSAID use. Indomethacin and ketorolac should not be used in older adults because of the increased risk of these gastrointestinal adverse effects.21 Concomitant use of NSAIDs and low-dose aspirin is associated with an increased risk of upper gastrointestinal bleeding.22
Studies of patients with rheumatoid arthritis or osteoarthritis, who were not taking low-dose aspirin and who did not have risk factors for gastrointestinal bleeding or peptic ulcers, have shown that celecoxib, NSAIDs, and acetaminophen have similar analgesic effects.23–25 Although celecoxib was associated with fewer gastrointestinal effects, the researchers concluded that this relatively small reduction does not justify the extra cost of celecoxib.24 Celecoxib alone and an NSAID plus a proton pump inhibitor (e.g., diclofenac plus omeprazole [Prilosec], naproxen plus lansoprazole [Prevacid]) have the same probability of causing recurrent ulcer bleeding or recurrent gastric or duodenal ulcer complications in those at high risk of these complications.26,27 However, a cost analysis suggests that in patients 75 years or older with a history of gastrointestinal bleeding or peptic ulcers, celecoxib treatment is less expensive than treatment with an NSAID plus misoprostol (Cytotec) or a proton pump inhibitor.24
In recent years, the concern about COX-2 selective NSAIDs and, to a lesser extent, nonselective NSAIDs has centered on their cardiovascular adverse effects (i.e., stroke, myocardial infarction, and thrombus formation),13 and these drugs include an FDA boxed warning regarding these risks. An analysis of six randomized, placebo-controlled trials evaluating the cardiovascular risk associated with celecoxib use showed that the risk increases with dose and that patients with higher baseline cardiovascular risk are more likely to experience a cardiovascular event while taking celecoxib.28 The cardiovascular risk is also thought to be greater with greater COX-2 selectivity (celecoxib > diclofenac > ibuprofen > naproxen).29
Renal insufficiency associated with NSAID use is related to inhibition of renal prostaglandin synthesis, which can present as azotemia and hyperkalemia. Use of NSAIDs in patients with impaired renal function, decreased creatinine clearance, or azotemia can result in acute renal failure.
Additional Pharmacotherapy
OPIOID COMBINATIONS
If nonopioid medications such as acetaminophen or NSAIDs do not adequately control pain, the next step of the WHO pain relief ladder includes considering an opioid, with or without a nonopioid.5 Opioids such as hydrocodone and oxycodone are typically combined with acetaminophen or an NSAID. In 2010, hydrocodone/acetaminophen was the most commonly dispensed medication in the United States.30 Opioid combinations are more effective than any one opioid for postoperative pain.14,15 In a meta-analysis of double-blind randomized controlled trials, patients who received an opioid, such as morphine, with an NSAID had significantly lower pain scores and needed significantly less of the opioid for pain control.31 Adding codeine, 60 mg, to acetaminophen, 600 to 1,000 mg, resulted in only 10% to 15% more patients achieving at least 50% pain relief compared with the same dose of acetaminophen alone.15
The FDA has been concerned about the potential for acetaminophen-induced hepatic injury,32 and in 2011 requested that manufacturers of prescription products limit the amount of acetaminophen in each dosage unit to no more than 325 mg and to include a boxed warning about the risk of severe hepatic injury.33 This does not affect over-the-counter products, although patients should not exceed 4,000 mg per day and should be cautioned about using prescription and over-the-counter products containing acetaminophen.
FULL OPIOID AGONISTS
Full opioid agonists, such as morphine, are potent analgesics that may be used if opioids combined with acetaminophen or NSAIDs are insufficient to control moderate to severe pain.14,15,31 There is a lack of good evidence to suggest any one opioid is more effective or has a better adverse effect profile than morphine.34 The exception is codeine. There is good evidence that codeine is less effective than morphine and other opioid agonists because of its low affinity for opioid receptors.35 Some opioids commonly used in the past are now unavailable (e.g., propoxyphene [Darvon]) or no longer widely used (e.g., meperidine [Demerol], pentazocine [Talwin]).
Adverse effects of opioids include nausea, vomiting, constipation, sedation, pruritus, urinary retention, and respiratory depression. Opioid-induced emesis is mediated by histamine release and can be treated with antihistamines or selective serotonin antagonists (e.g., ondansetron [Zofran]), if needed. Opioids rarely cause gastroparesis, which can present as persistent vomiting. If the opioid cannot be discontinued, opioid-induced gastroparesis can be treated with a gastric motility agent, such as metoclopramide (Reglan). However, caution should be used because metoclopramide can cause extrapyramidal adverse effects. There is no good evidence that adverse effects vary among the different opioids given at equianalgesic doses.36 If the initial opioid does not provide adequate pain relief or the patient experiences intolerable adverse effects, trying an alternative opioid may be reasonable. However, this has not been well studied in acute noncancer pain.37
DUAL-ACTION MEDICATIONS
Tapentadol (Nucynta), a Schedule II controlled substance, is a muopioid receptor agonist and norepinephrine reuptake inhibitor that can be used orally for relief of moderate to severe acute pain. It has similar effectiveness as oxycodone for acute pain, with a significantly lower incidence of nausea, vomiting, and constipation.38 Tapentadol should be used cautiously with serotonergic medications because of the risk of serotonin syndrome.
Tramadol (Ultram) is not federally controlled but is a Schedule IV controlled substance in some states; its designation is currently being reviewed. It is a weak muopioid receptor agonist and a weak inhibitor of norepinephrine and serotonin reuptake in the central nervous system. It is effective for acute pain (e.g., osteoarthritis) in clinical trials, but the benefits are small and it is considered a second-tier medication.16,39 Among patients presenting to the emergency department with acute musculoskeletal pain, hydrocodone/acetaminophen, 5 mg/500 mg, provided statistically and clinically significant reductions in pain at all time points compared with tramadol, 100 mg.39 Tramadol should be used cautiously, if at all, in patients at risk of seizures.
Diversion and Addiction Risks
Opioids should be used cautiously because of the risk of diversion and addiction, even with short-term use. A study showed that in 2010, 2 million persons used prescription pain relievers nonmedically in the prior year; this was second only to marijuana.40 Among individuals who reported the nonmedical use of a prescription analgesic, 55% obtained the drug from a friend or relative, 79% of whom obtained the prescription from a physician, and another 17% obtained the prescription directly from a physician.40 Among patients hospitalized for opioid dependence, 51% first started using the drug to treat pain (e.g., after a surgery, dental procedure, or injury).41 Patients should be counseled to safely dispose of any unused medication.42
Data Sources: A PubMed search was completed in Clinical Queries using the key terms pain, analgesics nonnarcotic, and analgesic narcotic. Also searched were the Cochrane database, Essential Evidence Plus, Physician's Information and Education Resource, and the National Guideline Clearinghouse. The search results included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: May and June 2011, and April and May 2012.
