
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2013;87(11):781-788
Patient information: See related handout on amenorrhea, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Although amenorrhea may result from a number of different conditions, a systematic evaluation including a detailed history, physical examination, and laboratory assessment of selected serum hormone levels can usually identify the underlying cause. Primary amenorrhea, which by definition is failure to reach menarche, is often the result of chromosomal irregularities leading to primary ovarian insufficiency (e.g., Turner syndrome) or anatomic abnormalities (e.g., Müllerian agenesis). Secondary amenorrhea is defined as the cessation of regular menses for three months or the cessation of irregular menses for six months. Most cases of secondary amenorrhea can be attributed to polycystic ovary syndrome, hypothalamic amenorrhea, hyperprolactinemia, or primary ovarian insufficiency. Pregnancy should be excluded in all cases. Initial workup of primary and secondary amenorrhea includes a pregnancy test and serum levels of luteinizing hormone, follicle-stimulating hormone, prolactin, and thyroid-stimulating hormone. Patients with primary ovarian insufficiency can maintain unpredictable ovarian function and should not be presumed infertile. Patients with hypothalamic amenorrhea should be evaluated for eating disorders and are at risk for decreased bone density. Patients with polycystic ovary syndrome are at risk for glucose intolerance, dyslipidemia, and other aspects of metabolic syndrome. Patients with Turner syndrome (or variant) should be treated by a physician familiar with the appropriate screening and treatment measures. Treatment goals for patients with amenorrhea may vary considerably, and depend on the patient and the specific diagnosis.
Many underlying conditions can lead to amenorrhea. Each of these conditions is associated with varying clinical sequelae; thus, it is important to consider a broad differential diagnosis to avoid missing rare or emergent pathology. Primary amenorrhea is defined as the failure to reach menarche. Evaluation should be undertaken if there is no pubertal development by 13 years of age, if menarche has not occurred five years after initial breast development, or if the patient is 15 years or older.1–3 Secondary amenorrhea is characterized as the cessation of previously regular menses for three months or previously irregular menses for six months.1,2 In contrast, a normal menstrual cycle typically occurs every 21 to 35 days.2
Primary amenorrhea is often, but not exclusively, the result of chromosomal irregularities that lead to primary ovarian insufficiency (e.g., Turner syndrome) or anatomic abnormalities (e.g., Müllerian agenesis). Most pathologic cases of secondary amenorrhea can be attributed to polycystic ovary syndrome (PCOS), hypothalamic amenorrhea, hyperprolactinemia, or primary ovarian insufficiency.1,4,5
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Pregnancy should be excluded in all patients presenting with amenorrhea. | C | 1, 2, 6, 7, 11 |
| In the evaluation of amenorrhea, hormone-induced withdrawal bleeding has poor sensitivity and specificity for ovarian function. | C | 1, 6, 13, 16, 17 |
| In patients with functional hypothalamic amenorrhea (especially with the female athlete triad), the primary treatment is weight restoration through nutritional rehabilitation and decreased exercise. | C | 7, 31, 32 |
| In patients with functional hypothalamic amenorrhea, combined oral contraceptives do not improve bone density and should not be used solely for this purpose. | C | 7, 31–36 |
| Patients with polycystic ovary syndrome who are overweight should be evaluated for glucose intolerance, dyslipidemia, and overall cardiovascular risk. | C | 1, 44 |
| Metformin (Glucophage) may improve abnormal menstruation in patients with polycystic ovary syndrome. | A | 44, 48–53 |
Evaluation
It is helpful to consider the possible causes of amenorrhea categorically. These include anatomic defects in the outflow tract; primary dysfunction of the ovary; disruption of hypothalamic or pituitary function; systemic disease affecting the hypothalamic-pituitary-gonadal axis; and pathology of other endocrine glands2 (Table 11,2,4–11 ).
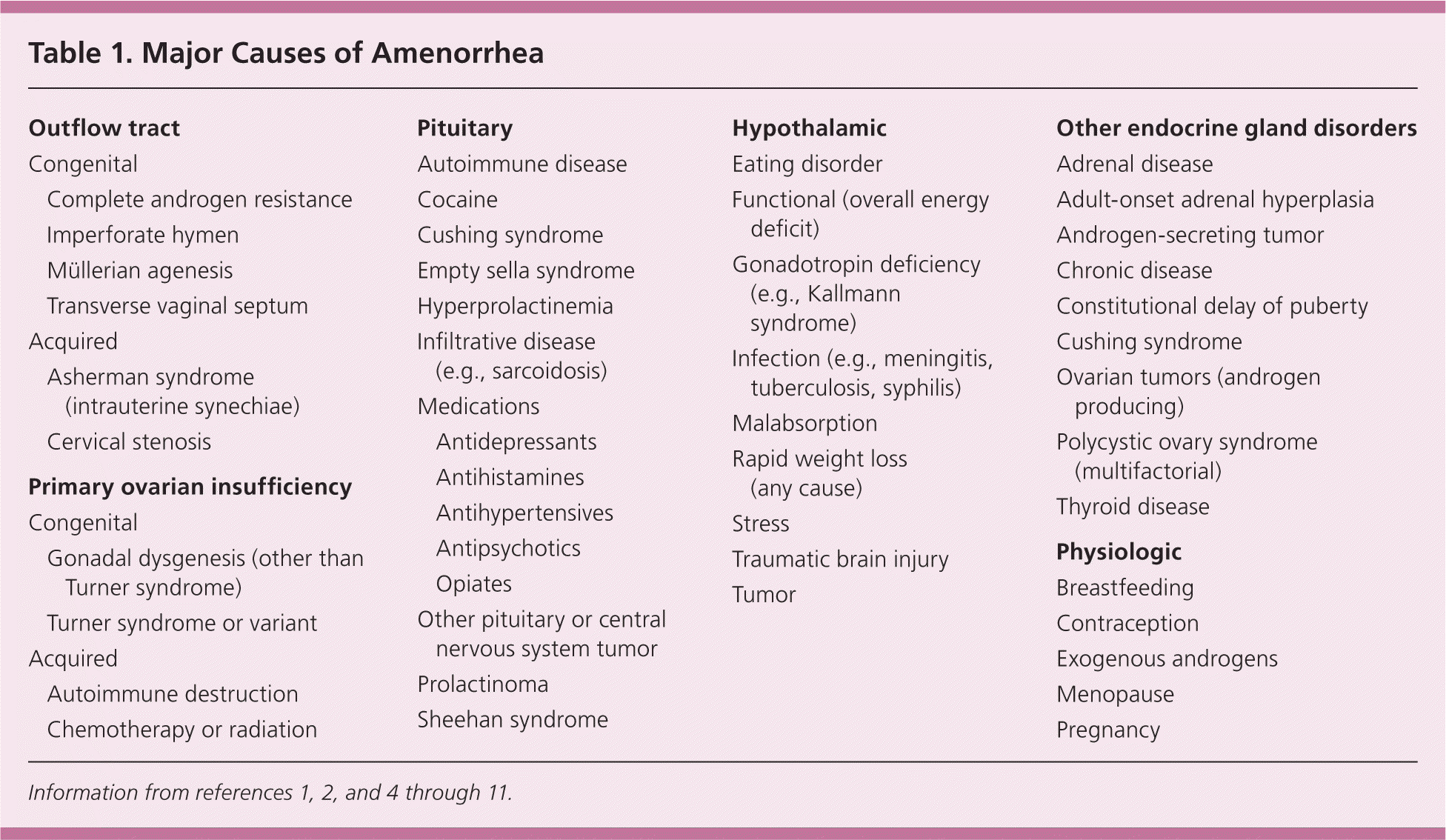
| Outflow tract | |
| Congenital | |
| Complete androgen resistance | |
| Imperforate hymen | |
| Müllerian agenesis | |
| Transverse vaginal septum | |
| Acquired | |
| Asherman syndrome (intrauterine synechiae) | |
| Cervical stenosis | |
| Primary ovarian insufficiency | |
| Congenital | |
| Gonadal dysgenesis (other than Turner syndrome) | |
| Turner syndrome or variant | |
| Acquired | |
| Autoimmune destruction | |
| Chemotherapy or radiation | |
| Pituitary | |
| Autoimmune disease | |
| Cocaine | |
| Cushing syndrome | |
| Empty sella syndrome | |
| Hyperprolactinemia | |
| Infiltrative disease (e.g., sarcoidosis) | |
| Medications | |
| Antidepressants | |
| Antihistamines | |
| Antihypertensives | |
| Antipsychotics | |
| Opiates | |
| Other pituitary or central nervous system tumor | |
| Prolactinoma | |
| Sheehan syndrome | |
| Hypothalamic | |
| Eating disorder | |
| Functional (overall energy deficit) | |
| Gonadotropin deficiency (e.g., Kallmann syndrome) | |
| Infection (e.g., meningitis, tuberculosis, syphilis) | |
| Malabsorption | |
| Rapid weight loss (any cause) | |
| Stress | |
| Traumatic brain injury | |
| Tumor | |
| Other endocrine gland disorders | |
| Adrenal disease | |
| Adult-onset adrenal hyperplasia | |
| Androgen-secreting tumor | |
| Chronic disease | |
| Constitutional delay of puberty | |
| Cushing syndrome | |
| Ovarian tumors (androgen producing) | |
| Polycystic ovary syndrome (multifactorial) | |
| Thyroid disease | |
| Physiologic | |
| Breastfeeding | |
| Contraception | |
| Exogenous androgens | |
| Menopause | |
| Pregnancy | |
A detailed history, examination, and laboratory analysis will identify most causes (Table 2).1,2,6,7,11 In all cases, pregnancy should first be excluded.1,2,6,7,11 The initial evaluative steps are similar; however, a major difference is the need to determine the presence or absence of the uterus in patients with primary amenorrhea (Figures 11,2,5–8,10,11 and 21,2,4,6–8,10,11). It is important to consider all causes of secondary amenorrhea in the evaluation of primary amenorrhea.
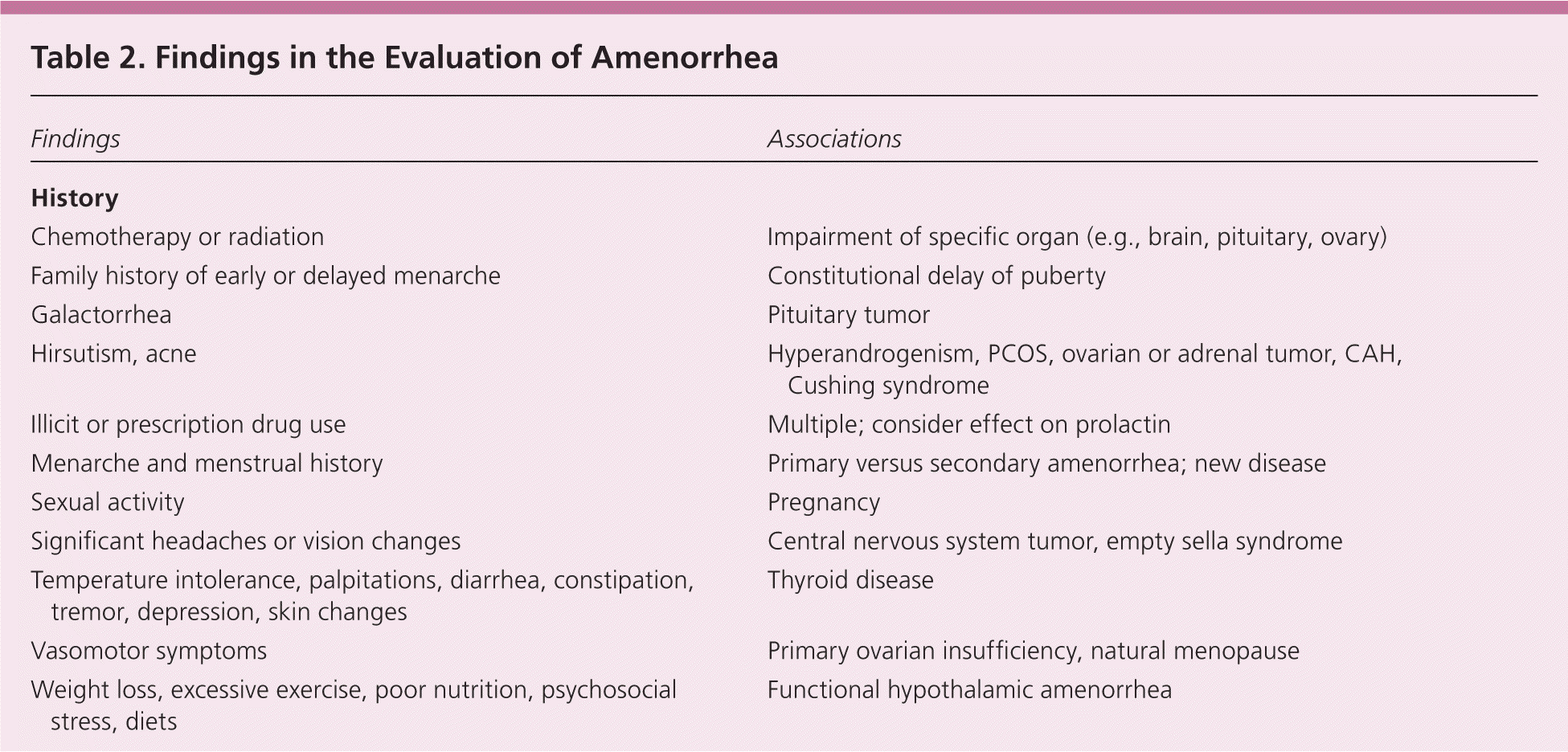
| Findings | Associations | |
|---|---|---|
| History | ||
| Chemotherapy or radiation | Impairment of specific organ (e.g., brain, pituitary, ovary) | |
| Family history of early or delayed menarche | Constitutional delay of puberty | |
| Galactorrhea | Pituitary tumor | |
| Hirsutism, acne | Hyperandrogenism, PCOS, ovarian or adrenal tumor, CAH, Cushing syndrome | |
| Illicit or prescription drug use | Multiple; consider effect on prolactin | |
| Menarche and menstrual history | Primary versus secondary amenorrhea; new disease | |
| Sexual activity | Pregnancy | |
| Significant headaches or vision changes | Central nervous system tumor, empty sella syndrome | |
| Temperature intolerance, palpitations, diarrhea, constipation, tremor, depression, skin changes | Thyroid disease | |
| Vasomotor symptoms | Primary ovarian insufficiency, natural menopause | |
| Weight loss, excessive exercise, poor nutrition, psychosocial stress, diets | Functional hypothalamic amenorrhea | |
| Physical examination | ||
| Abnormal thyroid examination | Thyroid disorder | |
| Anthropomorphic measurements; growth charts | Multiple; Turner syndrome, constitutional delay of puberty | |
| High: PCOS | ||
| Body mass index | ||
| Low: Functional hypothalamic amenorrhea | ||
| Dysmorphic features (webbed neck, short stature, low hairline) | Turner syndrome | |
| Male pattern baldness, increased facial hair, acne | Hyperandrogenism, PCOS, ovarian or adrenal tumor, CAH, Cushing syndrome | |
| Pelvic examination | ||
| Absence or abnormalities of cervix or uterus | Rare congenital causes | |
| Clitoromegaly | Androgen-secreting tumor, CAH | |
| Presence of transverse vaginal septum or imperforate hymen | Outflow tract obstruction | |
| Reddened or thin vaginal mucosa | Decreased endogenous estrogen | |
| Striae, buffalo hump, central obesity, hypertension | Cushing syndrome | |
| Tanner staging abnormal | Turner syndrome, constitutional delay of puberty, rare causes | |
| Laboratory testing (refer to local reference values) | ||
| Complete blood count and metabolic panel abnormalities | Chronic disease | |
| Estradiol | Low: Poor endogenous estrogen production (suggestive of poor ovarian function) | |
| Follicle-stimulating hormone and luteinizing hormone | High: Primary ovarian insufficiency, Turner syndrome | |
| Low: Functional hypothalamic amenorrhea | ||
| Normal: PCOS, Asherman syndrome, multiple others | ||
| Free and total testosterone; dehydroepiandrosterone sulfate | High: Hyperandrogenism, PCOS, ovarian or adrenal tumor, CAH, Cushing syndrome | |
| Karyotype | Abnormal: Turner syndrome, rare chromosomal disorders | |
| Pregnancy test | Positive: Pregnancy, ectopic pregnancy | |
| Prolactin | High: Pituitary adenoma, medications, hypothyroidism, other neoplasm | |
| Thyroid-stimulating hormone | High: Hypothyroidism | |
| Low: Hyperthyroidism | ||
| Diagnostic imaging | ||
| Magnetic resonance imaging of head or sella | Tumor (e.g., microadenoma) | |
| Pelvic ultrasonography | Morphology of pelvic organs | |
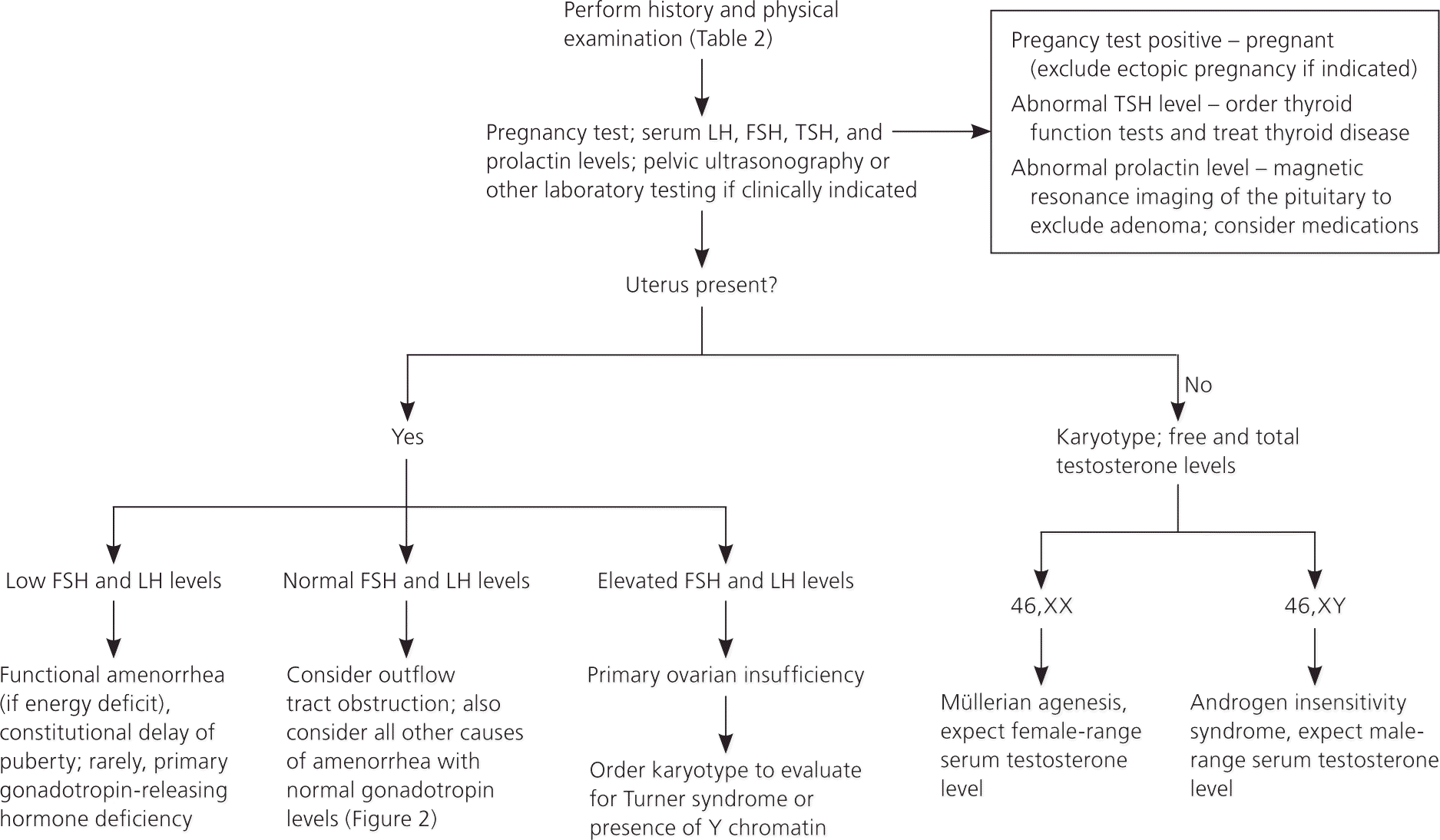
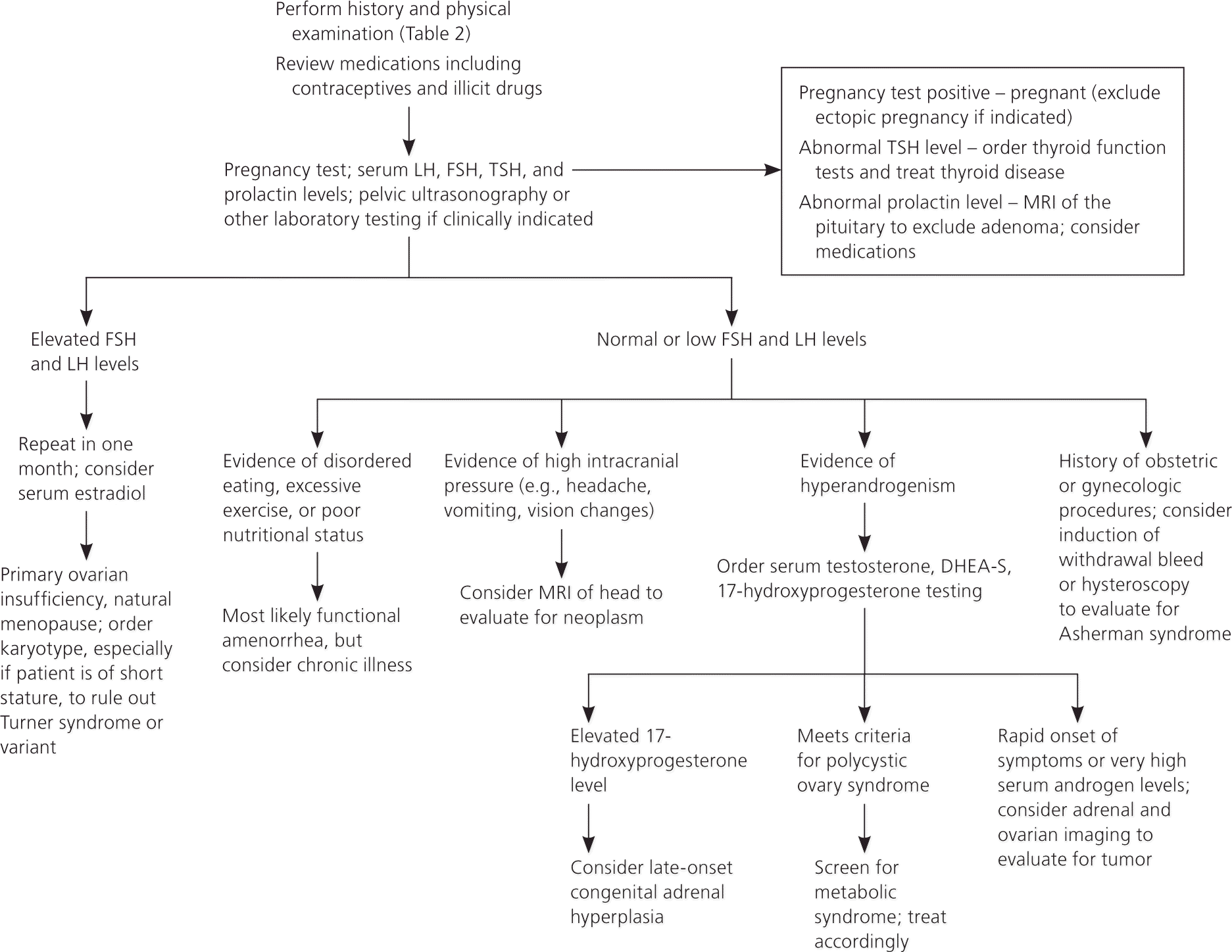
HISTORY
Patients should be asked about eating and exercise patterns, changes in weight, previous menses (if any), medication use, chronic illness, presence of galactorrhea, and symptoms of androgen excess, abnormal thyroid function, or vasomotor instability. Taking a sexual history can help corroborate the results of, but not replace, the pregnancy test. Family history should include age at menarche and presence of chronic disease. Although it is normal for menses to be irregular in the first few years after menarche, the menstrual interval is not usually longer than 45 days.7,12
PHYSICAL EXAMINATION
The physician should measure the patient's height, weight, and body mass index, and perform thyroid palpation and Tanner staging. Breast development is an excellent marker for ovarian estrogen production.1 Acne, virilization, or hirsutism may suggest hyperandrogenemia. Genital examination may reveal virilization, evidence of an outflow tract obstruction, or a missing or malformed organ. Thin vaginal mucosa is suggestive of low estrogen.7 Dysmorphic features such as a webbed neck or low hairline may suggest Turner syndrome.13
LABORATORY EVALUATION
The initial workup includes a pregnancy test and serum luteinizing hormone, follicle-stimulating hormone, prolactin, and thyroid-stimulating hormone levels. If history or examination suggests a hyperandrogenic state, serum free and total testosterone and dehydroepiandrosterone sulfate concentrations are useful.14 If the patient is short in stature, a karyotype analysis should be performed to exclude Turner syndrome.1,15 If the presence of endogenous estradiol secretion is not evident from the physical examination (e.g., breast development), serum estradiol may be measured.7 A complete blood count and comprehensive metabolic panel may be useful if history or examination is suggestive of chronic disease.7
FURTHER TESTING
Pelvic ultrasonography can help confirm the presence or absence of a uterus, and can identify structural abnormalities of reproductive tract organs. If a pituitary tumor is suspected, magnetic resonance imaging (MRI) may be indicated.8 Hormonal challenge (e.g., medroxyprogesterone acetate [Provera], 10 mg orally per day for seven to 10 days) with anticipation of a withdrawal bleed to confirm functional anatomy and adequate estrogenization, has traditionally been central to the evaluation.2 Some experts defer this testing because its correlation with estrogen status is relatively unreliable.1,6,13,16,17
Differential Diagnosis and Treatment
ANATOMIC ABNORMALITIES
Müllerian agenesis, a condition characterized by a congenital malformation of the genital tract, may present with normal breast development without menarche, and may be associated with urinary tract defects and fused vertebrae.18 Other congenital abnormalities that may cause amenorrhea include imperforate hymen and transverse vaginal septum. In these conditions, products of menstruation accumulate behind the defect and can lead to cyclic or acute pelvic pain. Physical examination, as well as ultrasonography or MRI, is key to diagnosis, and surgical correction is usually warranted.18
Rare causes of amenorrhea include complete androgen insensitivity syndrome, which is characterized by normal breast development, sparse or absent pubic and axillary hair, and a blind vaginal pouch; and 5-alpha reductase deficiency, which is characterized by partially virilized genitalia.1 In these conditions, serum testosterone levels will be in the same range as those found in males of the same age.19 The karyotype will be 46,XY, and testicular tissue should be removed to avoid malignant transformation.20
PRIMARY OVARIAN INSUFFICIENCY
Primary ovarian insufficiency, a condition characterized by follicle depletion or dysfunction leading to a continuum of impaired ovarian function, is suggested by a concentration of follicle-stimulating hormone in the menopausal range (per reference laboratory), confirmed on two occasions separated by one month, and diagnosed in patients younger than 40 years with amenorrhea or oligomenorrhea.6 Other terms, including premature ovarian failure, are used synonymously with primary ovarian insufficiency.6,9 Up to 1% of women may experience primary ovarian insufficiency. This condition differs from menopause, in which the average age is 50 years, because of age and less long-term predictability in ovarian function.6,22,23 More than 90% of cases unrelated to a syndrome are idiopathic, but they can be attributed to radiation, chemotherapeutic agents, infection, tumor, empty sella syndrome, or an autoimmune or infiltrative process.6
Patients with primary ovarian insufficiency should be counseled about possible fertility, because up to 10% of such patients may achieve temporary and unpredictable remission.24 [ corrected] Hormone therapy (e.g., 100 mcg of daily transdermal estradiol or 0.625 mg of daily conjugated equine estrogen [Premarin] on days 1 through 26 of the menstrual cycle, and 10 mg of cyclic medroxyprogesterone acetate for 12 days [e.g., days 14 through 26] of the menstrual cycle)6 until the average age of natural menopause is usually recommended to decrease the likelihood of osteoporosis, ischemic heart disease, and vasomotor symptoms.9 Combined oral contraceptives (OCs) deliver higher concentrations of estrogen and progesterone than necessary for hormone therapy, may confer thromboembolic risk, and may theoretically be ineffective at suppressing follicle-stimulating hormone for contraceptive purposes in this population; thus, a barrier method or intrauterine device is appropriate in sexually active patients.6,13,25,26 For optimal bone health, patients with primary ovarian insufficiency should be advised to perform weightbearing exercises and supplement calcium (e.g., 1,200 mg daily) and vitamin D3 (e.g., 800 IU daily) intake.6,27
Turner syndrome, a condition characterized by a chromosomal pattern of 45,X or a variant, can present with a classic phenotype including a webbed neck, a low hairline, cardiac defects, and lymphedema.13,15 Some patients who have Turner syndrome have only short stature and variable defects in ovarian function (even with possible fertility).6,13,15 Thus, all patients with short stature and amenorrhea should have a karyotype analysis.15 Because patients require screening for a number of systemic problems, including coarctation of the aorta, other cardiac lesions, renal abnormalities, hearing problems, and hypothyroidism, and because they may require human growth hormone treatment and hormone replacement therapy, physicians inexperienced with Turner syndrome should consult an endocrinologist.13,15
HYPOTHALAMIC AND PITUITARY CAUSES
The ovaries require physiologic stimulation by pituitary gonadotropins for appropriate follicular development and estrogen production. Functional hypothalamic amenorrhea occurs when the hypothalamic-pituitary-ovarian axis is suppressed due to an energy deficit stemming from stress, weight loss (independent of original weight), excessive exercise, or disordered eating.1,7 It is characterized by a low estrogen state without other organic or structural disease. Laboratory tests usually reveal low or low-normal levels of serum follicle-stimulating hormone, luteinizing hormone, and estradiol; however, these levels can fluctuate, and the clinical context is the discriminating factor.1,7 Patients with functional amenorrhea may demonstrate the features of the female athlete triad, which consists of insufficient caloric intake with or without an eating disorder, amenorrhea, and low bone density or osteoporosis.31 These patients should be screened for eating disorders, diets, and malabsorption syndromes (e.g., celiac disease).1
Treatment of functional hypothalamic amenorrhea involves nutritional rehabilitation as well as reductions in stress and exercise levels.7 Menses typically return after correction of the underlying nutritional deficit.32 Bone loss is best treated by reversal of the underlying process, and the patient should undergo bone density evaluation and take calcium and vitamin D supplements.7 Although the bone loss is partly secondary to estrogen deficiency, estrogen replacement without nutritional rehabilitation does not reverse the bone loss. Combined OCs will restore menses, but will not correct bone density.7,31,33–36 Leptin administration has been reported to restore pulsatility of gonadotropin-releasing hormone and ovulation in these patients, but its effect on bone health is unknown.7,37,38 The effect of bisphosphonates on long-term bone health in premenopausal women is unclear, as is their teratogenic potential.7,39
ELEVATIONS IN SERUM PROLACTIN
Prolactin levels can be elevated because of medications (Table 21,2,6,7,11 ), pituitary adenoma, hypothyroidism, or mass lesion compromising normal hypothalamic inhibition.8 Elevated prolactin levels, whatever the cause, inhibit the secretion and effect of gonadotropins, and warrant MRI of the pituitary.8 Exceptions may occur in cases with a clear pharmacologic trigger and relatively low levels of serum prolactin (i.e., < 100 ng per mL [< 100 mcg per L]).8 Treatment of prolactinomas may involve dopamine agonists or surgical resection.8
OTHER CENTRAL NERVOUS SYSTEM ETIOLOGIES
Amenorrhea can be caused by previous central nervous system infection, trauma, or autoimmune destruction of the pituitary.1 A notable consideration in primary amenorrhea is constitutional delay of puberty, a diagnosis of exclusion that is difficult to distinguish from functional amenorrhea without history of an energy deficit. Although rare, primary gonadotropin-releasing hormone deficiency, such as occurs with Kallmann syndrome (including anosmia), must be considered.40
Other Causes of Amenorrhea
POLYCYSTIC OVARY SYNDROME
PCOS is a multifactorial endocrine disorder, usually involving peripheral insulin resistance. It is characterized by hyperandrogenism found on clinical or laboratory examination, polycystic ovaries as suggested by ultrasonography, and ovulatory dysfunction. The Rotterdam Consensus Criteria published in 2003 require the presence of two of the three above conditions for diagnosis, whereas the Androgen Excess Society's 2006 guidelines require hyperandrogenism and either of the remaining two conditions.41,42 In PCOS, serum androgen levels are typically no greater than twice the upper limit of normal. Thus, higher levels suggest other causes of hyperandrogenism1,14,43 (Figure 21,2,4,6–8,10,11 ).
With insulin resistance contributing to the underlying pathology of PCOS, patients should be screened for dyslipidemia and overall cardiovascular risk. Glucose intolerance should be assessed with a fasting glucose and two-hour glucose tolerance test, because patients may have insulin resistance and beta-cell dysfunction.1,44 In patients with PCOS who are overweight, weight loss combined with exercise is the first-line treatment.44 Chronic anovulation with resultant unopposed estrogen secretion is a risk factor for endometrial cancer, and low-dose combined OCs are more frequently prescribed to reduce this risk than higher-dose pills or progestin-only methods.44–46 Many combined OCs suppress the secretion of ovarian androgen and may be useful in decreasing hirsutism and acne, although data are limited.10,44,47 Metformin (Glucophage) can increase insulin sensitivity, thereby improving glucose tolerance. It may also improve ovulation rate, reduce the incidence of menstrual abnormalities, and improve serum androgen concentrations.44,48–53
PREGNANCY AND CONTRACEPTION
All evaluations for amenorrhea should begin with a pregnancy test. If abdominal pain is present, ectopic pregnancy should be considered. Patients should be questioned about contraceptive use, because extended-cycle combined OCs, injectable medroxyprogesterone acetate (Depo-Provera), implantable etonogestrel (Implanon), and levonorgestrel-releasing intrauterine devices (Mirena) may cause amenorrhea.10
THYROID AND ADRENAL DISEASE
Late-onset congenital adrenal hyperplasia, androgen-secreting tumor, and Cushing syndrome must be distinguished from PCOS in the evaluation of hyperandrogenic amenorrhea. A high serum 17-hydroxyprogesterone level measured at 7:00 a.m. suggests congenital adrenal hyperplasia, which can be confirmed with an adrenocorticotropin stimulation test.14 An adrenal or ovarian tumor should be considered with rapid onset of symptoms or when serum androgens are significantly elevated, although cutoff levels are nonspecific.14,43 Rarely, hypercortisolism from Cushing syndrome may result in amenorrhea, and can be evaluated by a dexamethasone suppression test when stigmata of disease are present 4,14,44 (Table 11,2,4–11 ).
Data Sources: A PubMed search was completed using the MeSH function with the key phrases amenorrhea, evaluation, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Essential Evidence Plus, the Cochrane Database of Systematic Reviews, the National Guideline Clearinghouse database, the U.S. Preventive Services Task Force Web site, Clinical Evidence, and UpToDate. Search date: August 1, 2011.
