
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2013;88(1):25-32
Author disclosure: No relevant financial affiliations.
Postexposure prophylaxis (PEP) is effective in preventing illness after potential or documented exposure to a variety of microbial pathogens and in reducing the risk of secondary spread of infection. Guidelines have been published by the Centers for Disease Control and Prevention and the Advisory Committee on Immunization Practices for proper use of PEP for bloodborne pathogens, for microorganisms transmitted by either airborne or droplet spread or through direct contact, and for infections acquired after traumatic injuries. Depending on the type of exposure, different forms of PEP are available, including vaccines, immune globulins, antibiotics, and antiviral medications. Physicians should assess a patient's potential need for PEP based on several factors, including the type of exposure, the timing and severity of illness in the source patient, the exposed person's susceptibility to infectious diseases of concern, and the relative risks and benefits of the PEP regimen in an individual situation. Immunity to certain infectious diseases can be ensured with prior infection or vaccination, and by serologic testing in patients with a negative or uncertain history. PEP should be given to persons exposed to index cases of pertussis and invasive meningococcal infection regardless of immunization history, and should be given following rabies and tetanus exposure regardless of the length of delay. In general, PEP should be given as soon as possible following a high-risk exposure. Persons exposed to bloodborne pathogens should have baseline testing for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus antibodies, and follow-up testing at six weeks, three months, and six months postexposure.
A number of interventions reduce the risk of acquiring an infectious disease after exposure. For example, age-appropriate immunization can prevent measles, mumps, rubella, varicella, influenza, pertussis, and hepatitis B virus infection.1,2 Immunization is particularly important for health care workers who are not only at high risk of exposure to communicable diseases, but who can also transmit infection to high-risk patients.3 In health care settings, adherence to standard precautions such as routine hand hygiene, correct use of personal protective equipment (e.g., gowns, gloves, masks, N95 respirators, eye protection), and appropriate use of isolation precautions can reduce the risk of infection.4,5 Persons exposed to an infectious disease should be evaluated promptly by a physician. Postexposure prophylaxis (PEP) is effective in preventing development of the disease and, in some cases, in reducing the risk of secondary transmission to other susceptible persons.
PEP is routinely recommended following exposure to a wide spectrum of viral and bacterial diseases. Thus, physicians should be familiar with established PEP protocols. Exposure to communicable diseases occurs in occupational and nonoccupational settings. The infectious agent can be transmitted by another person, an animal, or the environment. Although many persons who seek medical care after an exposure visit an emergency department, others go to their primary care physician's office or, for health care workers, employee health service centers.6,7 The primary goals of the evaluation are to provide PEP if indicated, to allay the exposed person's anxiety, and to avoid unnecessary interventions and loss of workdays. In this article, we review infectious diseases for which PEP is indicated to prevent occupational or nonoccupational transmission, and discuss guidelines for PEP published by the Advisory Committee on Immunization Practices and other health agencies. A systematic approach to management will be described.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Selecting appropriate patients to receive postexposure prophylaxis should be based on the assessment of the type of exposure, the status of the source patient, and the status of the exposed person. | C | 8–10, 12, 14, 15, 17, 18, 24, 26, 27, 30 |
| Postexposure prophylaxis for common infectious diseases should be implemented according to published guidelines. For complex cases, consider consulting persons who have experience in infectious diseases or infection control. | C | 8–12, 14, 15, 17, 18, 21, 24, 26, 27, 30, 35 |
| For maximal effectiveness, postexposure prophylaxis for common infectious diseases should be started as early as possible and within the recommended period of administration. | C | 8–10, 14, 15, 17, 18, 24, 26, 28, 30 |
| After a patient has been exposed to bloodborne pathogens, the physician should obtain baseline testing for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus antibodies, and repeat testing in six weeks, three months, and six months. | C | 8, 9, 14 |
Determine if the Source Patient Is Infected with a Communicable Disease
When evaluating a person who may have been exposed to an infection, the first step is to assess the nature and source of the exposure. This can be accomplished by taking a history, performing a physical examination, and ordering appropriate laboratory testing. For certain infections, such as human immunodeficiency virus (HIV), hepatitis A virus, hepatitis B virus, and tuberculosis, the diagnosis must be confirmed microbiologically in the source patient by culture or serology to determine appropriate management.6,8–12
Determine if the Source Patient Is Infectious at the Time of Exposure
The infectious period is defined as the time frame in which others may have been exposed to an infectious disease while the source patient was contagious (Tables 1 through 33,8–29 ). [ corrected] Ask the source patient about the date of onset of illness and the last date of symptoms to estimate the infectious period and to identify potential contacts. Effective treatment can shorten the duration of microbial shedding and subsequently reduce transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and plague.12,24,30
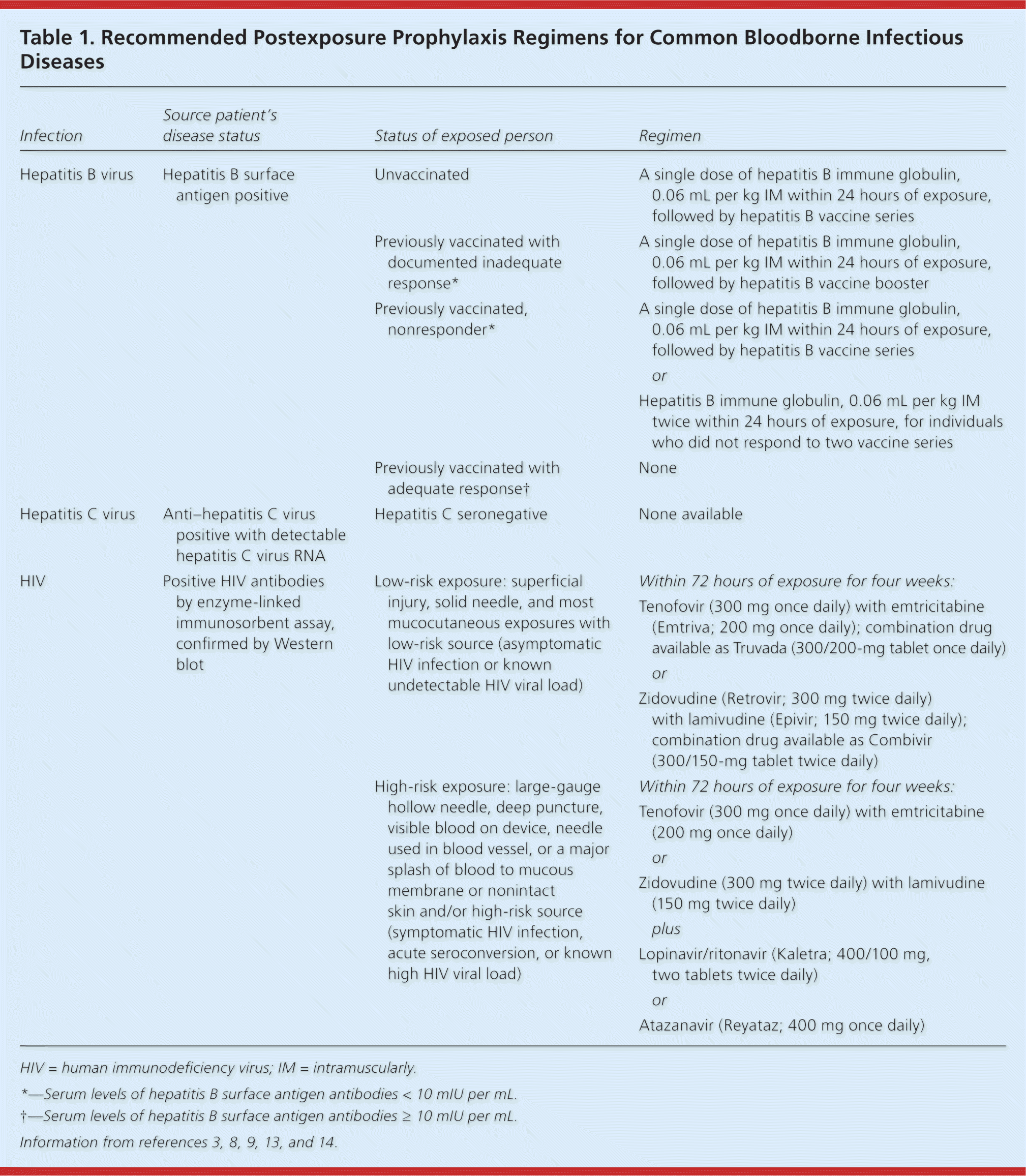
| Infection | Source patient's disease status | Status of exposed person | Regimen | |
|---|---|---|---|---|
| Hepatitis B virus | Hepatitis B surface antigen positive | Unvaccinated | A single dose of hepatitis B immune globulin, 0.06 mL per kg IM within 24 hours of exposure, followed by hepatitis B vaccine series | |
| Previously vaccinated with documented inadequate response* | A single dose of hepatitis B immune globulin, 0.06 mL per kg IM within 24 hours of exposure, followed by hepatitis B vaccine booster | |||
| Previously vaccinated, nonresponder* | A single dose of hepatitis B immune globulin, 0.06 mL per kg IM within 24 hours of exposure, followed by hepatitis B vaccine series | |||
| or | ||||
| Hepatitis B immune globulin, 0.06 mL per kg IM twice within 24 hours of exposure, for individuals who did not respond to two vaccine series | ||||
| Previously vaccinated with adequate response† | None | |||
| Hepatitis C virus | Anti–hepatitis C virus positive with detectable hepatitis C virus RNA | Hepatitis C seronegative | None available | |
| HIV | Positive HIV antibodies by enzyme-linked immunosorbent assay, confirmed by Western blot | Low-risk exposure: superficial injury, solid needle, and most mucocutaneous exposures with low-risk source (asymptomatic HIV infection or known undetectable HIV viral load) | Within 72 hours of exposure for four weeks: | |
| Tenofovir (300 mg once daily) with emtricitabine (Emtriva; 200 mg once daily); combination drug available as Truvada (300/200-mg tablet once daily) | ||||
| or | ||||
| Zidovudine (Retrovir; 300 mg twice daily) with lamivudine (Epivir; 150 mg twice daily); combination drug available as Combivir (300/150-mg tablet twice daily) | ||||
| High-risk exposure: large-gauge hollow needle, deep puncture, visible blood on device, needle used in blood vessel, or a major splash of blood to mucous membrane or nonintact skin and/or high-risk source(symptomatic HIV infection, acute seroconversion, or known high HIV viral load) | Within 72 hours of exposure for four weeks: | |||
| Tenofovir (300 mg once daily) with emtricitabine (200 mg once daily) | ||||
| or | ||||
| Zidovudine (300 mg twice daily) with lamivudine (150 mg twice daily) | ||||
| plus | ||||
| Lopinavir/ritonavir (Kaletra; 400/100 mg, two tablets twice daily) | ||||
| or | ||||
| Atazanavir (Reyataz; 400 mg once daily) | ||||
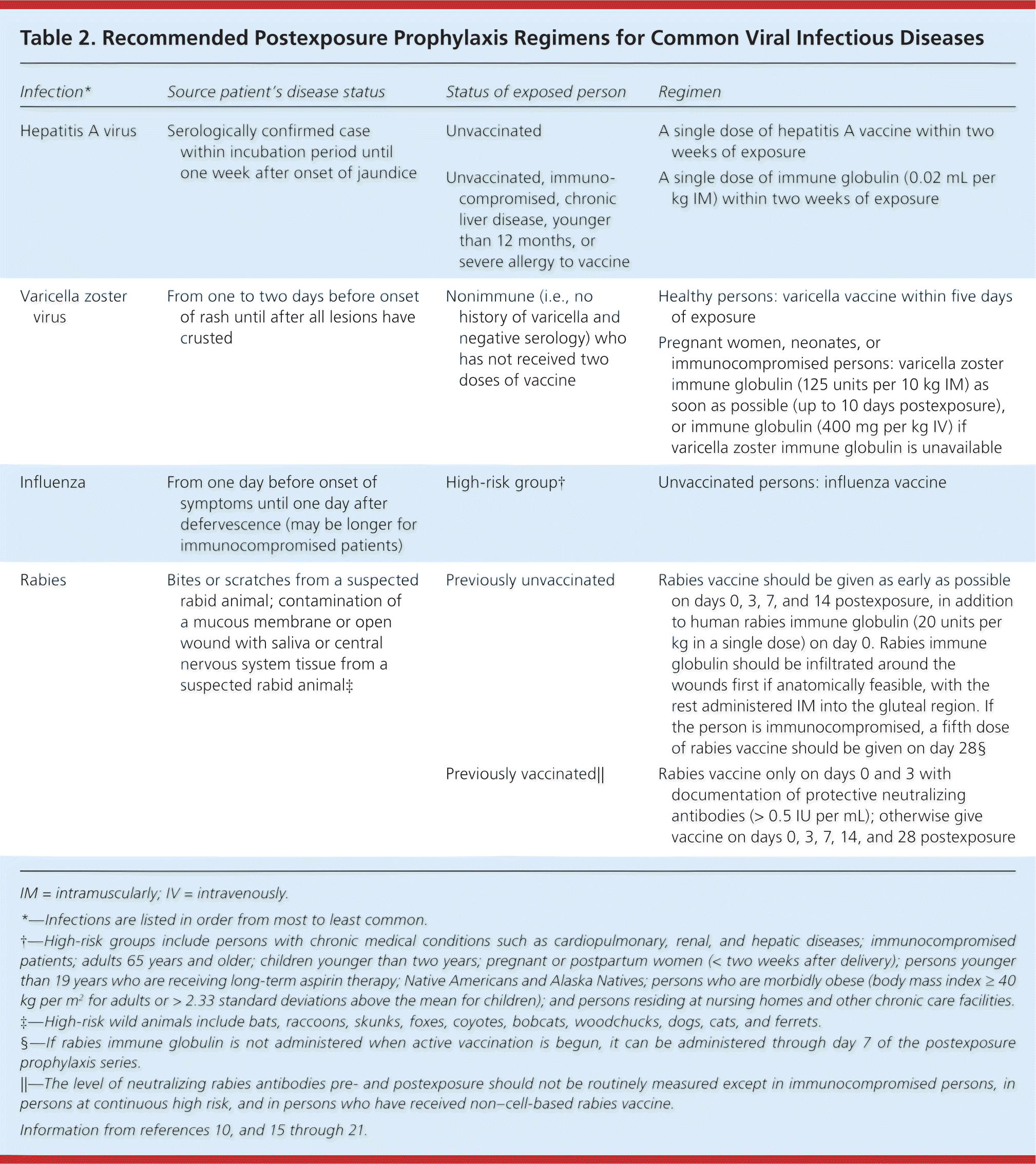
| Infection* | Source patient's disease status | Status of exposed person | Regimen |
|---|---|---|---|
| Hepatitis A virus | Serologically confirmed case within incubation period until one week after onset of jaundice | Unvaccinated | A single dose of hepatitis A vaccine within two weeks of exposure |
| Unvaccinated, immunocompromised, chronic liver disease, younger than 12 months, or severe allergy to vaccine | A single dose of immune globulin (0.02 mL per kg IM) within two weeks of exposure | ||
| Varicella zoster virus | From one to two days before onset of rash until after all lesions have crusted | Nonimmune (i.e., no history of varicella and negative serology) who has not received two doses of vaccine | Healthy persons: varicella vaccine within five days of exposure |
| Pregnant women, neonates, or immunocompromised persons: varicella zoster immune globulin (125 units per 10 kg IM) as soon as possible (up to 10 days postexposure), or immune globulin (400 mg per kg IV) if varicella zoster immune globulin is unavailable | |||
| Influenza | From one day before onset of symptoms until one day after defervescence (may be longer for immunocompromised patients) | High-risk group† | Unvaccinated persons: influenza vaccine |
| Rabies | Bites or scratches from a suspected rabid animal; contamination of a mucous membrane or open wound with saliva or central nervous system tissue from a suspected rabid animal‡ | Previously unvaccinated | Rabies vaccine should be given as early as possible on days 0, 3, 7, and 14 postexposure, in addition to human rabies immune globulin (20 units per kg in a single dose) on day 0. Rabies immune globulin should be infiltrated around the wounds first if anatomically feasible, with the rest administered IM into the gluteal region. If the person is immunocompromised, a fifth dose of rabies vaccine should be given on day 28.§ |
| Previously vaccinated|| | Rabies vaccine only on days 0 and 3 with documentation of protective neutralizing antibodies (> 0.5 IU per mL); otherwise give vaccine on days 0, 3, 7, 14, and 28 postexposure |
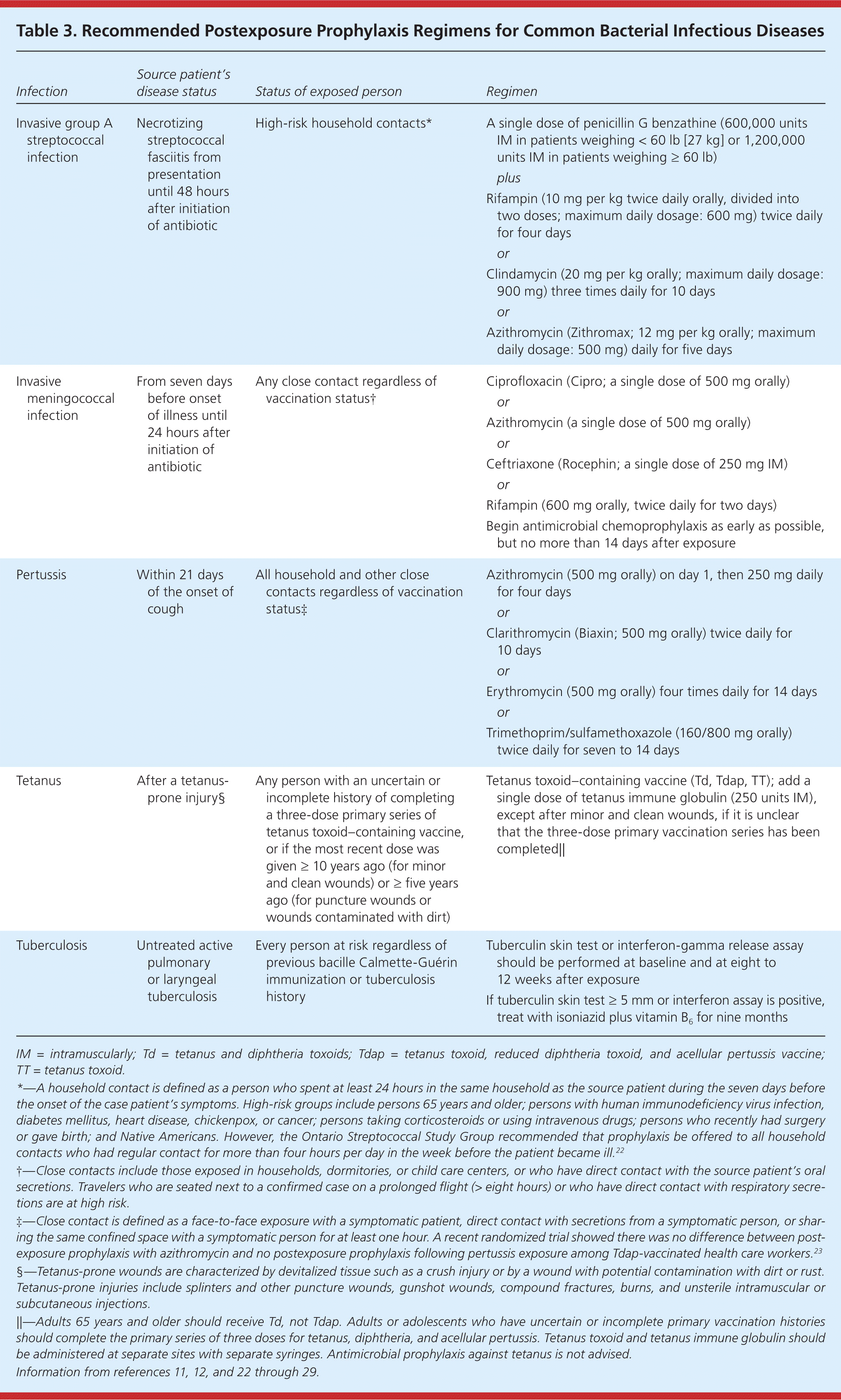
| Infection | Source patient's disease status | Status of exposed person | Regimen | |
|---|---|---|---|---|
| Invasive group A streptococcal infection | Necrotizing streptococcal fasciitis from presentation until 48 hours after initiation of antibiotic | High-risk household contacts* | A single dose of penicillin G benzathine (600,000 units IM in patients weighing < 60 lb [27 kg] or 1,200,000 units IM in patients weighing = 60 lb) | |
| plus | ||||
| Rifampin (10 mg per kg twice daily orally, divided into two doses; maximum daily dosage: 600 mg) twice daily for four days | ||||
| or | ||||
| Clindamycin (20 mg per kg orally; maximum daily dosage: 900 mg) three times daily for 10 days | ||||
| or | ||||
| Azithromycin (Zithromax; 12 mg per kg orally; maximum daily dosage: 500 mg) daily for five days | ||||
| Invasive meningococcal infection | From seven days before onset of illness until 24 hours after initiation of antibiotic | Any close contact regardless of vaccination status† | Ciprofloxacin (Cipro; a single dose of 500 mg orally) | |
| or | ||||
| Azithromycin (a single dose of 500 mg orally) | ||||
| or | ||||
| Ceftriaxone (Rocephin; a single dose of 250 mg IM) | ||||
| or | ||||
| Rifampin (600 mg orally, twice daily for two days) | ||||
| Begin antimicrobial chemoprophylaxis as early as possible, but no more than 14 days after exposure | ||||
| Pertussis | Within 21 days of the onset of cough | All household and other close contacts regardless of vaccination status‡ | Azithromycin (500 mg orally) on day 1, then 250 mg daily for four days | |
| or | ||||
| Clarithromycin (Biaxin; 500 mg orally) twice daily for10 days | ||||
| or | ||||
| Erythromycin (500 mg orally) four times daily for 14 days | ||||
| or | ||||
| Trimethoprim/sulfamethoxazole (160/800 mg orally) twice daily for seven to 14 days | ||||
| Tetanus | After a tetanus-prone injury§ | Any person with an uncertain or incomplete history of completing a three-dose primary series of tetanus toxoid–containing vaccine, or if the most recent dose was given ≥ 10 years ago (for minor and clean wounds) or ≥ five years ago (for puncture wounds or wounds contaminated with dirt) | Tetanus toxoid–containing vaccine (Td, Tdap, TT); add a single dose of tetanus immune globulin (250 units IM), except after minor and clean wounds, if it is unclear that the three-dose primary vaccination series has been completed|| | |
| Tuberculosis | Untreated active pulmonary or laryngeal tuberculosis | Every person at risk regardless of previous bacille Calmette-Guérin immunization or tuberculosis history | Tuberculin skin test or interferon-gamma release assay should be performed at baseline and at eight to 12 weeks after exposure | |
| If tuberculin skin test ≥ 5 mm or interferon assay is positive, for nine months treat with isoniazid plus vitamin B6 | ||||
Determine the Type and Characteristics of the Exposure
A significant exposure to an infectious disease is defined as one in which the risk of transmitting microorganisms is relatively high (Table 44,6,31 ). For example, hepatitis B virus, hepatitis C virus, and HIV can be transmitted by percutaneous injuries from needles or other sharp objects, or by contact of mucous membranes or injured skin with blood or other infectious body fluids. Urine, feces, saliva, sputum, and vomitus, unless mixed with blood, are not considered infectious for bloodborne pathogens.9,13 Factors that affect the risk of transmission for bloodborne pathogens include the type, frequency, and severity of exposure; the type of body fluid involved; the microorganism; the volume of exposed fluid entering the body; the viral concentration of the fluid; and the status of the source person.4,8–10,12,14,15,17,18,24,26,27,30,31 For bloodborne pathogens, the average risk of infection after percutaneous exposure to blood from a patient infected with HIV is approximately 0.3%32; for hepatitis B virus, 6% (if the hepatitis B e antigen is negative) to 31% (if the hepatitis B e antigen is positive)33; and for hepatitis C virus, 1.8%.13,34
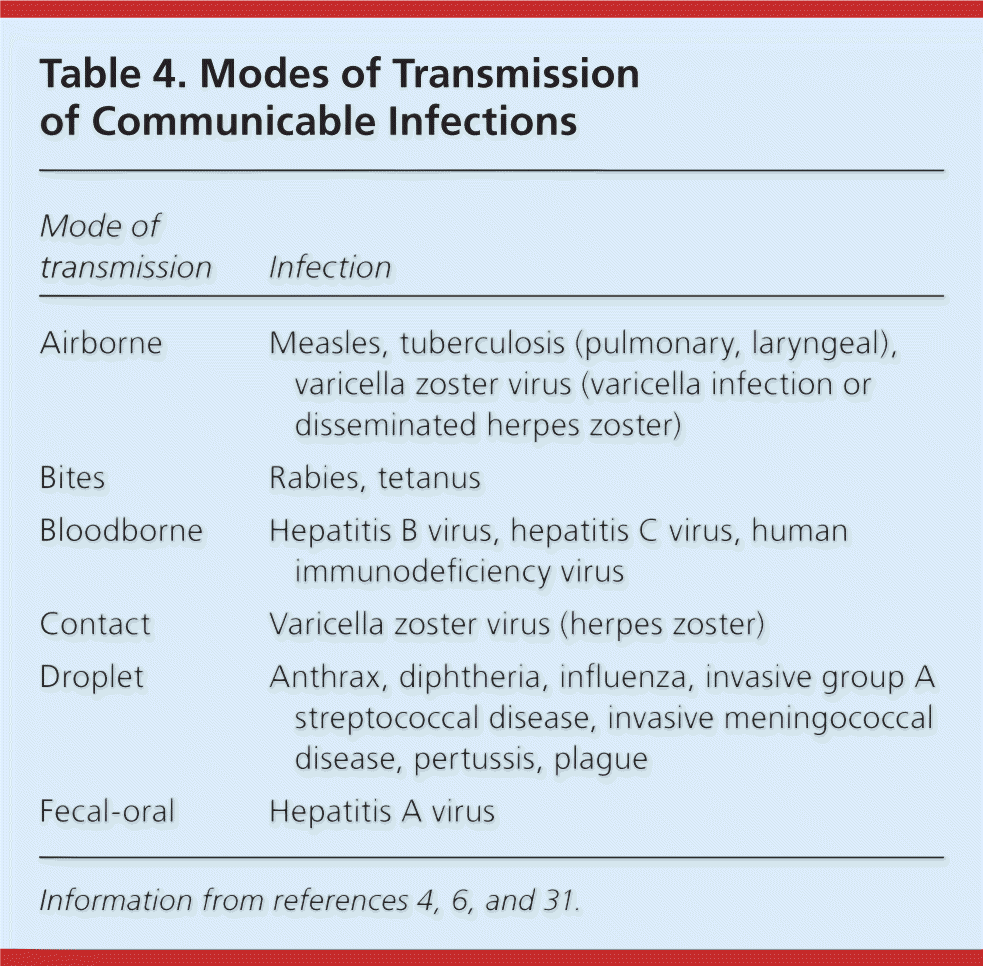
| Mode of transmission | Infection |
|---|---|
| Airborne | Measles, tuberculosis (pulmonary, laryngeal), varicella zoster virus (varicella infection or disseminated herpes zoster) |
| Bites | Rabies, tetanus |
| Bloodborne | Hepatitis B virus, hepatitis C virus, human immunodeficiency virus |
| Contact | Varicella zoster virus (herpes zoster) |
| Droplet | Anthrax, diphtheria, influenza, invasive group A streptococcal disease, invasive meningococcal disease, pertussis, plague |
| Fecal-oral | Hepatitis A virus |
Certain infectious diseases can be transmitted by airborne route when there is close or prolonged contact to a person with an active disease and when appropriate infection control precautions are not followed.1–4,11,12,15,31,35 Droplet transmission occurs when mucous membranes come in contact with an infected person's respiratory secretions if appropriate infection control precautions are not followed.1–4,16,22,24–27,30,31,36
Determine if the Exposed Person Is Susceptible
Persons exposed to an infectious disease should be evaluated within hours of the exposure, if possible. To determine whether the exposed patient has immunity to certain infectious diseases, a history of infection or immunization should be obtained, or serologic testing should be performed if the history is negative or uncertain24,26(Tables 1 through 33,8–29 ). PEP should be given to persons exposed to index cases of pertussis and invasive meningococcal infection regardless of immunization history, and should be given following rabies and tetanus exposure regardless of the length of delay. If the exposed person was wearing appropriate personal protective equipment or adhering to standard infection control practices at the time of exposure, then transmission is unlikely and PEP is not required.4,5,31
Determine the Proper Management for Exposure, Including PEP Regimen
PEP for common infectious diseases should be implemented according to published guidelines. For complex cases, consider consulting persons who have experience in infectious diseases or infection control.8–12,14,15,17,18,21,24,26,27,30,35 After percutaneous injury with a used needle or other sharp instrument, soap and water should be used to clean the wound. There is no evidence that applying antiseptics or disinfectants is beneficial9; use of bleach should be avoided. After mucosal exposure to blood or body fluids, the exposed area should be irrigated copiously with water. For the PEP regimen to be beneficial, it should be safe, effective, and affordable. For maximal effectiveness, PEP for common infectious diseases should be started as early as possible and within the recommended period of administration8–10,14,15,17,18,24,26,28,30 (Tables 1 through 33,8–29 ). However, for certain infections, such as rabies and tetanus, prophylaxis can be given up to several months after exposure because the incubation period is variable.17,25,28 PEP regimens for rare infectious diseases are available online (eTable A).
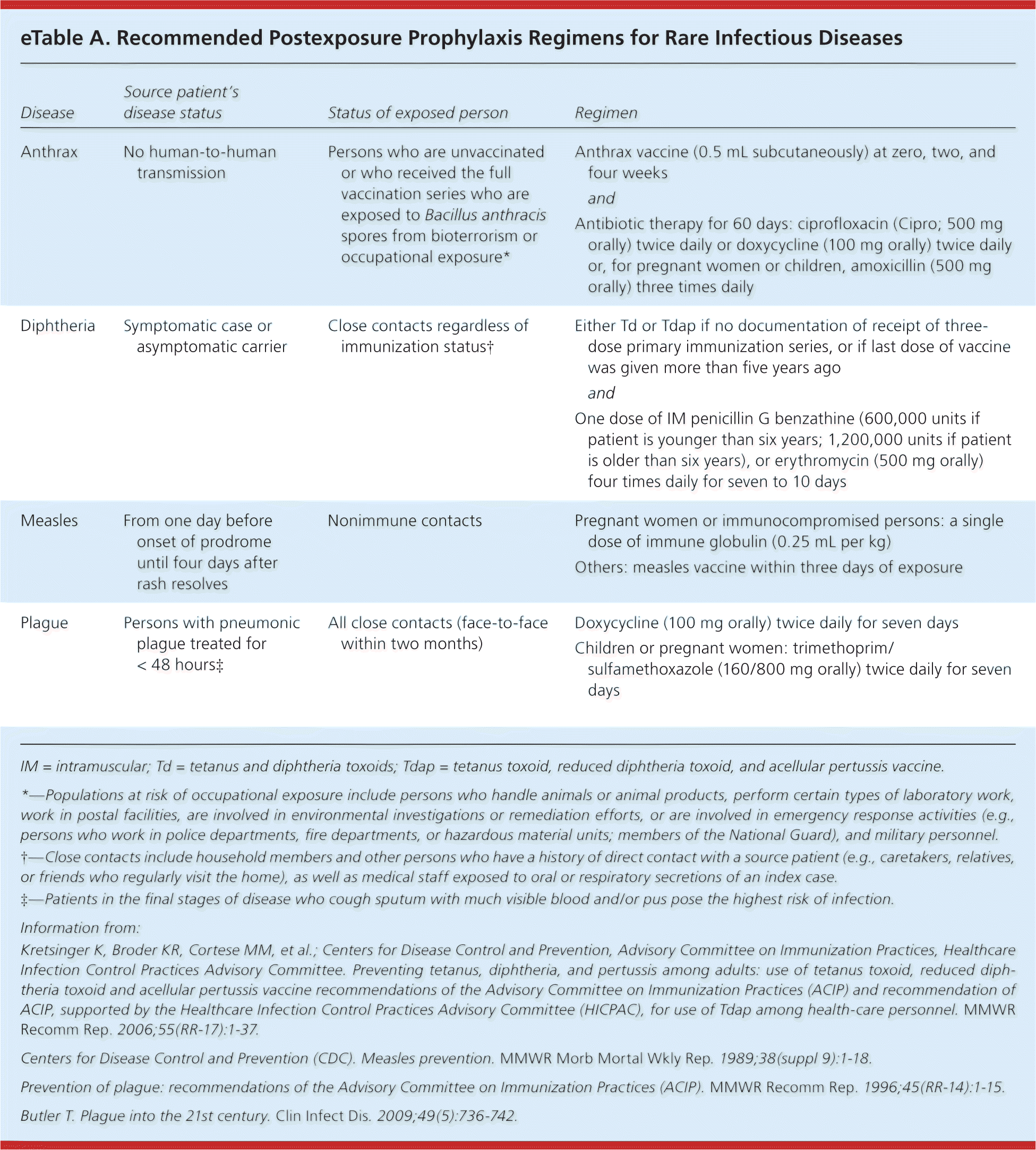
| Disease | Source patient's disease status | Status of exposed person | Regimen |
|---|---|---|---|
| Anthrax | No human-to-human transmission | Persons who are unvaccinated or who received the full vaccination series who are exposed to Bacillus anthracis spores from bioterrorism or occupational exposure* | Anthrax vaccine (0.5 mL subcutaneously) at zero, two, and four weeks |
| and | |||
| Antibiotic therapy for 60 days: ciprofloxacin (Cipro; 500 mg orally) twice daily or doxycycline (100 mg orally) twice daily or, for pregnant women or children, amoxicillin (500 mg orally) three times daily | |||
| Diphtheria | Symptomatic case or asymptomatic carrier | Close contacts regardless of immunization status† | Either Td or Tdap if no documentation of receipt of three-dose primary immunization series, or if last dose of vaccine was given more than five years ago |
| and | |||
| One dose of IM penicillin G benzathine (600,000 units if patient is younger than six years; 1,200,000 units if patient is older than six years), or erythromycin (500 mg orally) four times daily for seven to 10 days | |||
| Measles | From one day before onset of prodrome until four days after rash resolves | Nonimmune contacts | Pregnant women or immunocompromised persons: a single dose of immune globulin (0.25 mL per kg) |
| Others: measles vaccine within three days of exposure | |||
| Plague | Persons with pneumonic plague treated for < 48 hours‡ | All close contacts (face-to-face within two months) | Doxycycline (100 mg orally) twice daily for seven days |
| Children or pregnant women: trimethoprim/sulfamethoxazole (160/800 mg orally) twice daily for seven days |
The PEP regimen can be an antimicrobial agent, a vaccine, an immune globulin preparation, or a combination of these18–20 (Tables 1 through 33,8–29 ). Live vaccines are contraindicated in immunocompromised patients and pregnant women. In these groups, immune globulins, if available, are the preferred PEP regimen.15,20,21 Despite the risk of transmission to an exposed person, certain infections cannot be prevented by PEP, including adenovirus conjunctivitis, hepatitis C virus, mumps, parvovirus B19, and rubella.6
Recommendations for PEP for HIV after occupational exposure are based on the source patient's HIV viral load and resistance pattern and the severity of the exposure,8,14,23,37,38 and are summarized in Table 1.3,8,9,13,14 Nonoccupational PEP, with the same regimens, is recommended within 72 hours following high-risk sexual activity or use of contaminated needles.14
Appropriate Baseline and Follow-Up Assessments
Persons exposed to an infectious source should be assessed at baseline and at subsequent intervals while they are at risk of developing the disease of concern (Table 58–12,14 ). After a patient has been exposed to blood-borne pathogens, the physician should obtain baseline testing for HIV, hepatitis B virus, and hepatitis C virus antibodies, and repeat testing at six weeks, three months, and six months8,9,14 (Table 58–12,14 ). Persons who receive chemoprophylaxis against bacterial pathogens should be monitored for signs and symptoms of infection and for possible adverse effects from drugs. Exposed persons should be followed until completion of their prophylaxis regimens, including immunizations. Management of exposures should be documented and exposures reported to the appropriate administrative department.
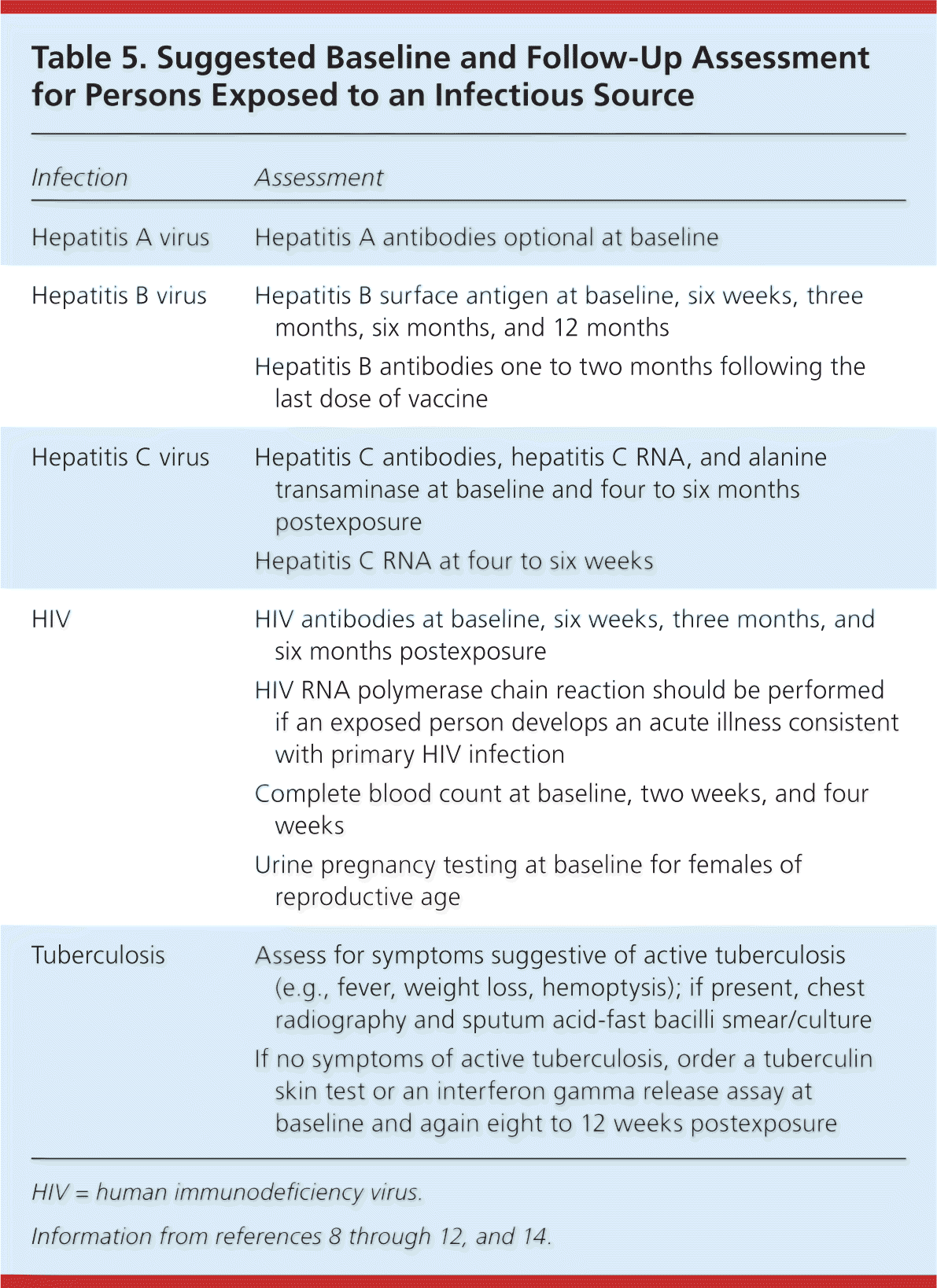
| Infection | Assessment |
|---|---|
| Hepatitis A virus | Hepatitis A antibodies optional at baseline |
| Hepatitis B virus | Hepatitis B surface antigen at baseline, six weeks, three months, six months, and 12 months |
| Hepatitis B antibodies one to two months following the last dose of vaccine | |
| Hepatitis C virus | Hepatitis C antibodies, hepatitis C RNA, and alanine transaminase at baseline and four to six months postexposure |
| Hepatitis C RNA at four to six weeks | |
| HIV | HIV antibodies at baseline, six weeks, three months, and six months postexposure |
| HIV RNA polymerase chain reaction should be performed if an exposed person develops an acute illness consistent with primary HIV infection | |
| Complete blood count at baseline, two weeks, and four weeks | |
| Urine pregnancy testing at baseline for females of reproductive age | |
| Tuberculosis | Assess for symptoms suggestive of active tuberculosis (e.g., fever, weight loss, hemoptysis); if present, chest radiography and sputum acid-fast bacilli smear/culture |
| If no symptoms of active tuberculosis, order a tuberculin skin test or an interferon-gamma release assay at baseline and again eight to 12 weeks postexposure |
Counseling the Exposed Person
Exposure to an infectious source, particularly to blood or body fluids, often produces anxiety about the possibility of disease acquisition and transmission. The patient or employee should be counseled about a number of issues, such as the confidentiality of all information (including test results); quantification of the potential risk of infection; the reasons for recommending (or not recommending) PEP; the possible adverse effects of PEP; and the need to avoid transmitting the disease to others. Counseling should be repeated on follow-up visits. Psychological support should be an integral part of counseling.6 Persons exposed to bloodborne viral pathogens should be told not to donate blood, plasma, or semen until follow-up testing has excluded seroconversion. In addition, persons known to have been exposed to HIV should avoid breastfeeding and should follow barrier precautions during sexual activity for a month until viral seroconversion has been excluded.9
Data Sources: We performed a PubMed search for reviews and clinical trials using the terms postexposure prophylaxis, hepatitis A, hepatitis B, hepatitis C, HIV, varicella, pertussis, diphtheria, influenza, rabies, group A streptococcus, anthrax, measles, mumps, Mycobacterium tuberculosis, tetanus, plague, and Neisseria meningitidis. We reviewed guidelines for postexposure prophylaxis published by the Advisory Committee on Immunization Practices and the Hospital Infection Control Practices Advisory Committee between 2000 and 2012. We also reviewed available guidelines from the Infectious Diseases Society of America pertaining to these infections. Essential Evidence Plus was utilized to validate the recommendations for postexposure prophylaxis. Search date: November 2012.
