Am Fam Physician. 2013;88(3):193-196
Patient information: See related handout on thyroid nodules, written by the author of this article.
Author disclosure: No relevant financial affiliations.
Thyroid nodules are a common finding in the general population. They may present with symptoms of pressure in the neck or may be discovered during physical examination. Although the risk of cancer is small, it is the main reason for workup of these lesions. Measurement of thyroid-stimulating hormone can identify conditions that may cause hyperfunctioning of the thyroid. For all other conditions, ultrasonography and fine-needle aspiration are central to the diagnosis. Lesions larger than 1 cm should be biopsied. Lesions with features suggestive of malignancy and those in patients with risk factors for thyroid cancer should be biopsied, regardless of size. Smaller lesions and those with benign histology can be followed and reevaluated if they grow. The evaluation of thyroid nodules in euthyroid and hypothyroid pregnant women is the same as in other adults. Thyroid nodules are uncommon in children, but the malignancy rate is much higher than in adults. Fine-needle aspiration is less accurate in children, so more aggressive surgical excision may be preferable.
Thyroid nodules are common in the general population, especially in women.1 Nonpalpable nodules are often found when patients undergo diagnostic imaging such as ultrasonography and computed tomography of the chest and neck. For these incidentalomas, current guidelines recommend the same diagnostic strategy that is recommended for palpable nodules.2 Although the risk of malignancy in any given nodule is small, thyroid cancer must be considered in the differential diagnosis. Family physicians should understand the rationale for the evaluation of nodules and be able to perform an evidence-based assessment.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The serum thyroid-stimulating hormone level should be measured during the initial evaluation of a patient with a thyroid nodule. If it is low, radionuclide scintigraphy should be performed. | C | 1, 14 |
| Thyroid ultrasonography should be performed in patients with known or suspected thyroid nodules. | C | 1, 14 |
| Fine-needle aspiration is the procedure of choice for sampling thyroid nodules for biopsy, except for hyperfunctioning nodules, which do not require biopsy. | C | 2 |
Epidemiology
Thyroid nodules can be palpated in 4% to 7% of adults.3 However, they are found incidentally in up to 40% of patients who undergo ultrasonography of the neck,4 and in 36% to 50% of persons at autopsy.3 Some studies estimate that 20% to 76% of the population has at least one thyroid nodule.3 The Framingham Study estimated the annual incidence of new palpable thyroid nodules to be 0.09%,5 which would have meant about 300,000 new nodules in U.S. patients in 2005. Because many more nodules can be detected with ultrasonography or computed tomography than can be palpated, the true incidence is much higher.6 Factors associated with increasing numbers and size of thyroid nodules include Graves disease7 and pregnancy.8 Low iodine intake is associated with an increased incidence of hyperfunctioning nodules (also called toxic adenomas).9
Thyroid cancer represents 1% of all malignancies.10 The rate of malignancy is 1.5% to 17% in nodules detected on imaging performed for non–thyroid-related reasons.4 However, the true rate of malignancy is unknown, because many nodules are small enough to escape detection, and because many malignancies in small nodules appear to have a benign course and do not cause clinically evident disease.6,7 Factors associated with increased risk of thyroid cancer include history of radiation to the head or neck, especially in childhood.3 The rate of malignancy for a palpable nodule in a previously irradiated thyroid is 20% to 50%.11 Nodules in persons younger than 20 years or older than 70 years have an increased risk of malignancy.3 Some studies have shown men to be at higher risk than women,3 and some suggest that thyroid cancer may be more common in patients with Graves disease.7 Family history may be an important factor. The rate of medullary thyroid carcinoma in persons with multiple endocrine neoplasia (MEN) type 2A or 2B is 25%,12 and rare cases of familial papillary thyroid carcinoma have been reported.13
Presentation
Thyroid nodules are often noticed by patients as a lump or protrusion in the lower anterior neck. Large nodules can cause compressive symptoms, such as difficulty swallowing or a choking sensation. Nodules may be single or multiple, hard or soft, and tender or nontender.
Nodules may also be found by physicians on routine examination. Clinical examination of the thyroid is difficult in persons with large necks. Nodules 1 cm or smaller are rarely detected by palpation.
Evaluation
The primary goal when evaluating a thyroid nodule is to determine whether it is malignant. Figure 1 presents a suggested algorithm for evaluating and treating thyroid nodules.14 Because of genetic mutations, a small number of nodules escape regulation by the normal thyroid-stimulating hormone (TSH) feedback system and autonomously produce thyroid hormone.15 Although reliable figures are not available, the proportion is probably about 5%.15 These so-called “hot” nodules are unlikely to be malignant and require treatment that is somewhat different than that for other nodules.
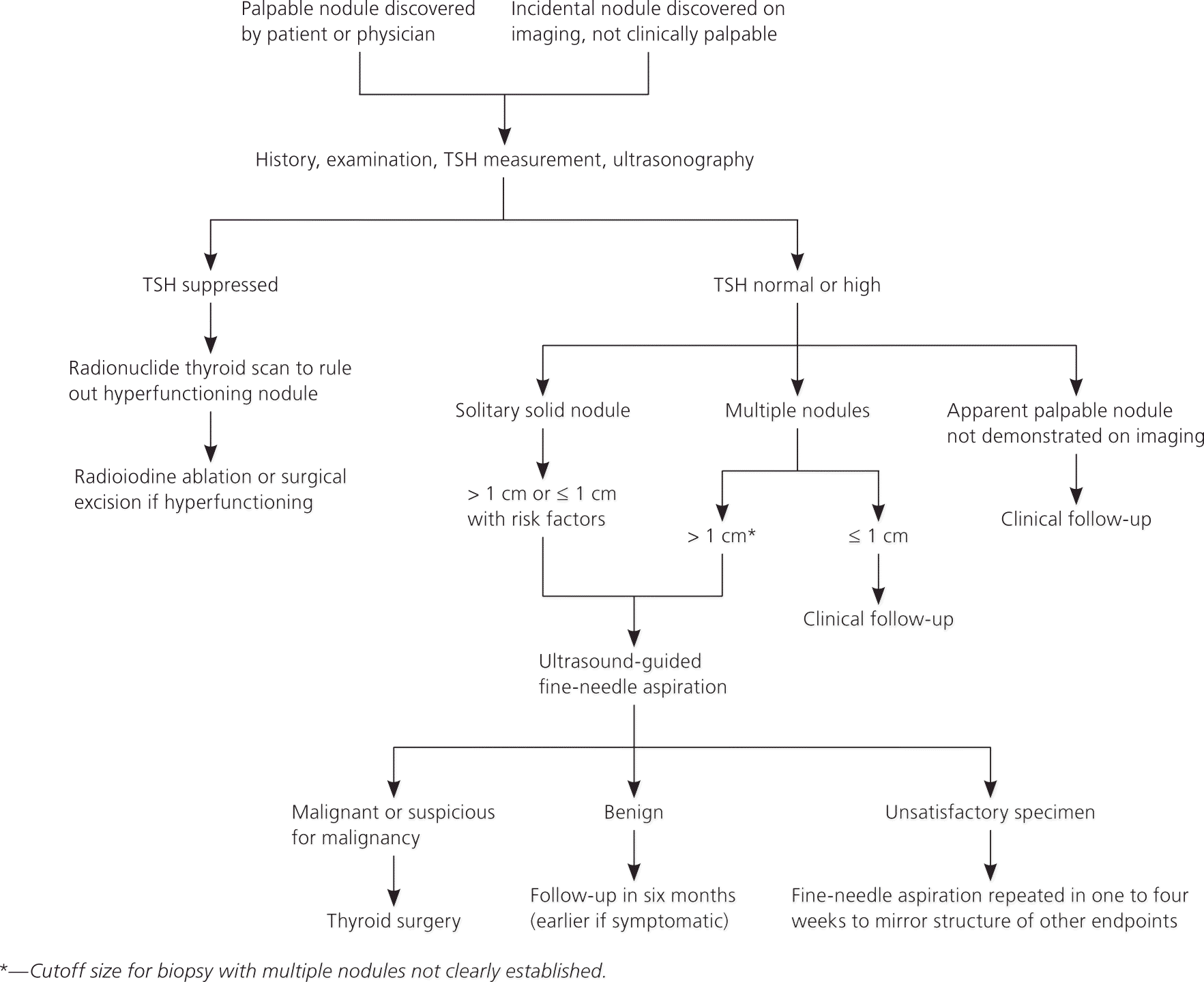
A reasonable first step in evaluating a thyroid nodule is to check TSH levels and perform thyroid ultrasonography.1,14 If TSH is suppressed, radionuclide scintigraphy with technetium 99m or iodine 123 can determine whether there are hyperfunctioning nodules or whether the entire thyroid gland is overactive, as it would be in cases of toxic multinodular goiter.1,14
In the past, nuclear thyroid scintigraphy was often performed to evaluate all thyroid nodules. However, nonfunctioning nodules have only a 14% to 22% chance of being malignant,16 and ultrasonography is now the imaging modality of choice.1,14 Although earlier guidelines recommended biopsy of smaller lesions, more recent recommendations are to biopsy only those larger than 1 cm.2 If a larger nodule is found, the next step is fine-needle aspiration (FNA). Nodules 1 cm or smaller may be followed with serial ultrasonography.2,11 More than one nodule should be biopsied if multiple nodules are found on ultrasonography.1,2,14 In such cases, there is no consensus on the recommended size for biopsy. It also has not been established how many nodules should be biopsied, but some authors suggest that sampling more than three is unnecessary.14 Nodules of any size should be biopsied if ultrasonography suggests extracapsular invasion by the lesion or shows cervical lymphadenopathy.2 Nodules also should be biopsied if the patient has a history of head and neck irradiation, thyroid cancer, or MEN type 2 in a first-degree relative.2 Hyperfunctioning nodules do not need to be biopsied.2
Larger nodules may be biopsied without ultrasound guidance, but the use of ultrasonography generally improves the diagnostic accuracy of FNA.17 Any nodule determined by FNA to be nondiagnostic or indeterminate should be reassessed with ultrasound guidance, if it was not used for the initial biopsy.17 Ultrasonography also should be used when sampling cystic lesions, because the target tissue is the solid component of the lesion.17
Most pathologists classify FNA specimens in one of four categories: malignant, suspicious, benign, and indeterminate or nondiagnostic. The malignant and benign categories are the most accurate, with false-negative rates of 1% to 10% and false-positive rates of about 2%.16 Suspicious samples have malignancy rates of 40% to 45%.16
Some experts advocate the measurement of serum calcitonin levels as part of the workup for thyroid nodules. Calcitonin levels are elevated in patients with medullary thyroid carcinoma. However, this disease is rare, and there is no clear threshold that distinguishes between benign and malignant disease.6 Previous guidelines found insufficient evidence to recommend for or against this practice,1,14 although more recent guidelines recommend measuring calcitonin in patients with thyroid nodules and a family history or clinical suspicion of medullary thyroid carcinoma or MEN type 2.2
Treatment
Radioactive iodine 131 ablation is the first-line treatment for hyperfunctioning thyroid nodules. Because activity in the surrounding tissue is suppressed, there is little uptake of the isotope in the tissue outside the nodules, and there does not seem to be any significant damage to the remainder of the thyroid.9 Studies in patients with Graves disease who were treated with radioactive iodine do not show a significant increase in the risk of mortality from thyroid cancer after treatment.7 Although thyroid cancer is more common after radioiodine treatment, these cancers tend to be less aggressive. Therefore, the overall thyroid cancer mortality rate is not increased.7
If the pathology is malignant or suspicious, surgery to remove the affected thyroid lobe or lobes is recommended.1,14 Diagnostic lobectomy is often recommended for nodules 4 cm or larger because this size is an independent predictor of malignancy, and because FNA in a large nodule may miss a malignant focus and be falsely interpreted as benign.3 Benign nodules should be followed with repeat ultrasonography six to 18 months after the initial FNA.1,2 If the nodules have not grown significantly at the follow-up examination, the interval may be extended to three to five years.1,2 If the nodule has grown, repeat FNA should be performed with ultrasound guidance.1,2 Recurrent cystic nodules with benign histology may be removed surgically or percutaneously injected with ethanol if they are symptomatic.1,2 Solid nodules that are benign on repeat FNA may be followed with ultrasonography or removed surgically, depending on symptoms.1,2 Studies of levothyroxine suppression in benign nodules have shown some reduction in nodule size,18 but this treatment is generally not recommended.1,2
Special Populations
Some studies show that the rate of development of thyroid nodules is higher in pregnant women than in non-pregnant women, but that the thyroid cancer rate is not increased during pregnancy.8 The evaluation of thyroid nodules in euthyroid and hypothyroid pregnant women is the same as in other adults, including ultrasonography and FNA when indicated.1 Levothyroxine suppression of growing nodules may be attempted, but the evidence of effectiveness is weak.1 In pregnant women with suppressed TSH levels, workup should be deferred until after pregnancy and lactation, so that thyroid scintigraphy can be performed.1 Symptomatic hyperthyroidism caused by hyperfunctioning nodules during pregnancy should be treated with antithyroid medications in the same manner as hyperthyroidism caused by Graves disease.19
Thyroid nodules in children are rare, occurring in about 1% to 2% of children.20 However, the malignancy rate in these nodules may be as high as 27%, much higher than in adults.20 The role of FNA in children is controversial because of uncertainty about its accuracy, although some studies report its accuracy to be as high as 90%.20 Some authors suggest that the accuracy of FNA in adolescents is about the same as that in adults, and recommend using it in these patients. FNA is less reliable in preadolescents, and excision of nodules rather than FNA is recommended.20 In children and adolescents with a family history of thyroid cancer or MEN type 2, aggressive early prophylactic thyroidectomy is recommended for treatment of thyroid nodules.20
Data Sources: An Ovid Medline search was completed using the key term thyroid nodule with the limits of human, English, full text, core clinical journals, and publication years 1995 to 2011. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search date: February 28, 2011.