
Am Fam Physician. 2013;88(4):269-275
Guideline source: American College of Obstetricians and Gynecologists
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Not stated
Published source: Obstetrics & Gynecology, September 2012
Available at: http://journals.lww.com/greenjournal/Citation/2012/09000/Practice_Bulletin_No__129___Osteoporosis.41.aspx (subscription required)
This practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) reviews diagnosis, evaluation, and treatment options for women with osteoporosis.
Recommendations
Women should be counseled on the recommended daily dietary allowances for calcium and vitamin D from the Institute of Medicine, which are as follows:
Persons nine to 18 years of age: 1,300 mg of calcium, 600 IU of vitamin D
Persons 19 to 50 years of age: 1,000 mg of calcium, 600 IU of vitamin D
Persons 51 to 70 years of age: 1,200 mg of calcium, 600 IU of vitamin D
Persons 71 years and older: 1,200 mg of calcium, 800 IU of vitamin D
A serum vitamin D level of 20 ng per mL (50 nmol per L) is recommended for good bone health.
Dual energy x-ray absorptiometry (DEXA) should be performed in all women 65 years and older. It can also be performed in postmenopausal women younger than 65 years who are at risk of fracture, including those with a history of fragility fracture, who weigh less than 127 lb (58 kg), who take medications or have diseases that cause bone loss, who have a parental history of hip fracture, who smoke, who have alcoholism, or who have rheumatoid arthritis. DEXA every one or two years can be used to determine the effectiveness of treatment; however, it should not be performed more often than every two years if the patient has no new risk factors, or if bone mineral density has improved or has not changed significantly.
In women older than 65 years with low bone mass, as indicated by bone mineral density reports, the World Health Organization's Fracture Risk Assessment Tool (FRAX; available at http://www.shef.ac.uk/FRAX/index.aspx) should be used to establish if a woman is at increased risk of fracture. Treatment is recommended if she is at increased risk. Among those not at increased risk, screening should be performed every 15 years for women older than 65 years with a normal bone mineral density or a T-score of −1.5 or greater, every five years for women with a T-score of −1.5 to −1.99, and every year for women with a T-score of −2.0 to −2.49.
Treatment is recommended in women with a T-score of −2.5 or less. For women with a T-score between −1.0 and −2.5, FRAX can assist in making an informed decision about treatment. Pharmacologic treatment should be considered in women with a 10-year risk of major osteoporotic fracture of at least 20% or a risk of hip fracture of at least 3%. Treatment should also be considered in women who have had a low-trauma fracture, even if DEXA does not indicate osteoporosis.
Because nutrition and lifestyle (e.g., inactivity, smoking) affect bone health, these factors should be discussed with females of all ages. To reduce the risk of bone loss and osteoporotic fractures, women with osteoporosis or who are at risk of osteoporosis should be counseled about lifestyle changes (e.g., weight-bearing exercise, smoking cessation, reducing alcohol intake).
First-line therapy usually consists of bisphosphonates; selection should be based on patient preference. Raloxifene (Evista) can be a good initial treatment in younger postmenopausal women, and denosumab is an option for women with a high risk of fracture. Teriparatide (Forteo) is typically only used in women with severe osteoporosis or who have had fractures. Calcitonin has weaker data compared with other options; therefore, it should be used only in women with less serious osteoporosis who cannot tolerate other treatments. Typically, combination therapy is not recommended. Whether there should be a limit to the duration of bisphosphonate therapy is unknown; however, there appears to be a trend toward interrupting therapy after five to 10 years. Hormone therapy (i.e., estrogen or combined estrogen/progestogen) positively affects bone health; it is approved for use in women with an increased risk of osteoporosis and fracture. Medications for preventing and treating postmenopausal osteoporosis are listed in Tables 1 and 2.
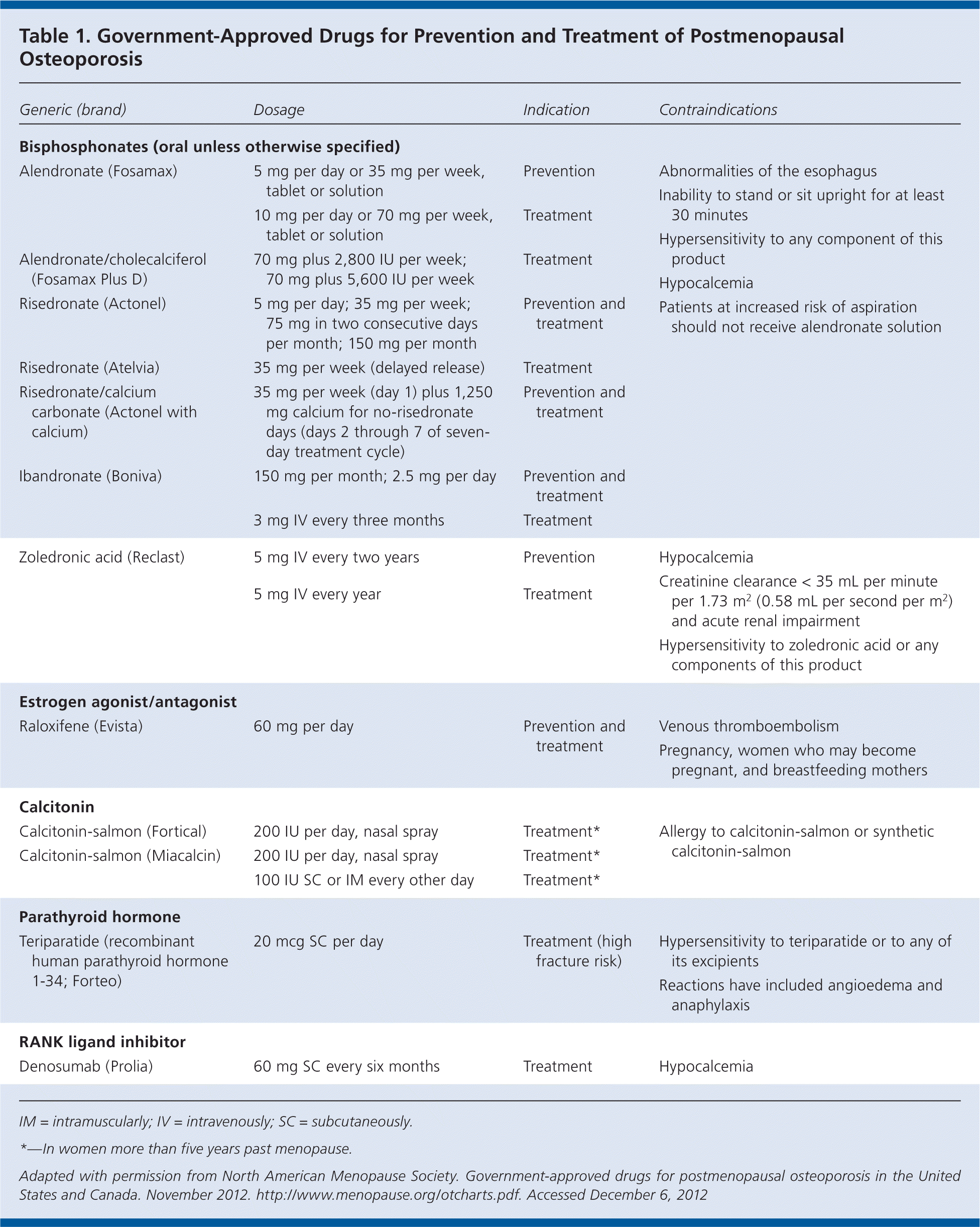
| Generic (brand) | Dosage | Indication | Contraindications |
|---|---|---|---|
| Bisphosphonates (oral unless otherwise specified) | |||
| Alendronate (Fosamax) | 5 mg per day or 35 mg per week, tablet or solution | Prevention |
|
| 10 mg per day or 70 mg per week, tablet or solution | Treatment | ||
| Alendronate/cholecalciferol (Fosamax Plus D) | 70 mg plus 2,800 IU per week; 70 mg plus 5,600 IU per week | Treatment | |
| Risedronate (Actonel) | 5 mg per day; 35 mg per week; 75 mg in two consecutive days per month; 150 mg per month | Prevention and treatment | |
| Risedronate (Atelvia) | 35 mg per week (delayed release) | Treatment | |
| Risedronate/calcium carbonate (Actonel with calcium) | 35 mg per week (day 1) plus 1,250 mg calcium for no-risedronate days (days 2 through 7 of seven-day treatment cycle) | Prevention and treatment | |
| Ibandronate (Boniva) | 150 mg per month; 2.5 mg per day | Prevention and treatment | |
| 3 mg IV every three months | Treatment | ||
| Zoledronic acid (Reclast) | 5 mg IV every two years | Prevention |
|
| 5 mg IV every year | Treatment | ||
| Estrogen agonist/antagonist | |||
| Raloxifene (Evista) | 60 mg per day | Prevention and treatment |
|
| Calcitonin | |||
| Calcitonin-salmon (Fortical) | 200 IU per day, nasal spray | Treatment* |
|
| Calcitonin-salmon (Miacalcin) | 200 IU per day, nasal spray | Treatment* | |
| 100 IU SC or IM every other day | Treatment* | ||
| Parathyroid hormone | |||
| Teriparatide (recombinant human parathyroid hormone1-34; Forteo) | 20 mcg SC per day | Treatment (high fracture risk) |
|
| RANK ligand inhibitor | |||
| Denosumab (Prolia) | 60 mg SC every six months | Treatment |
|
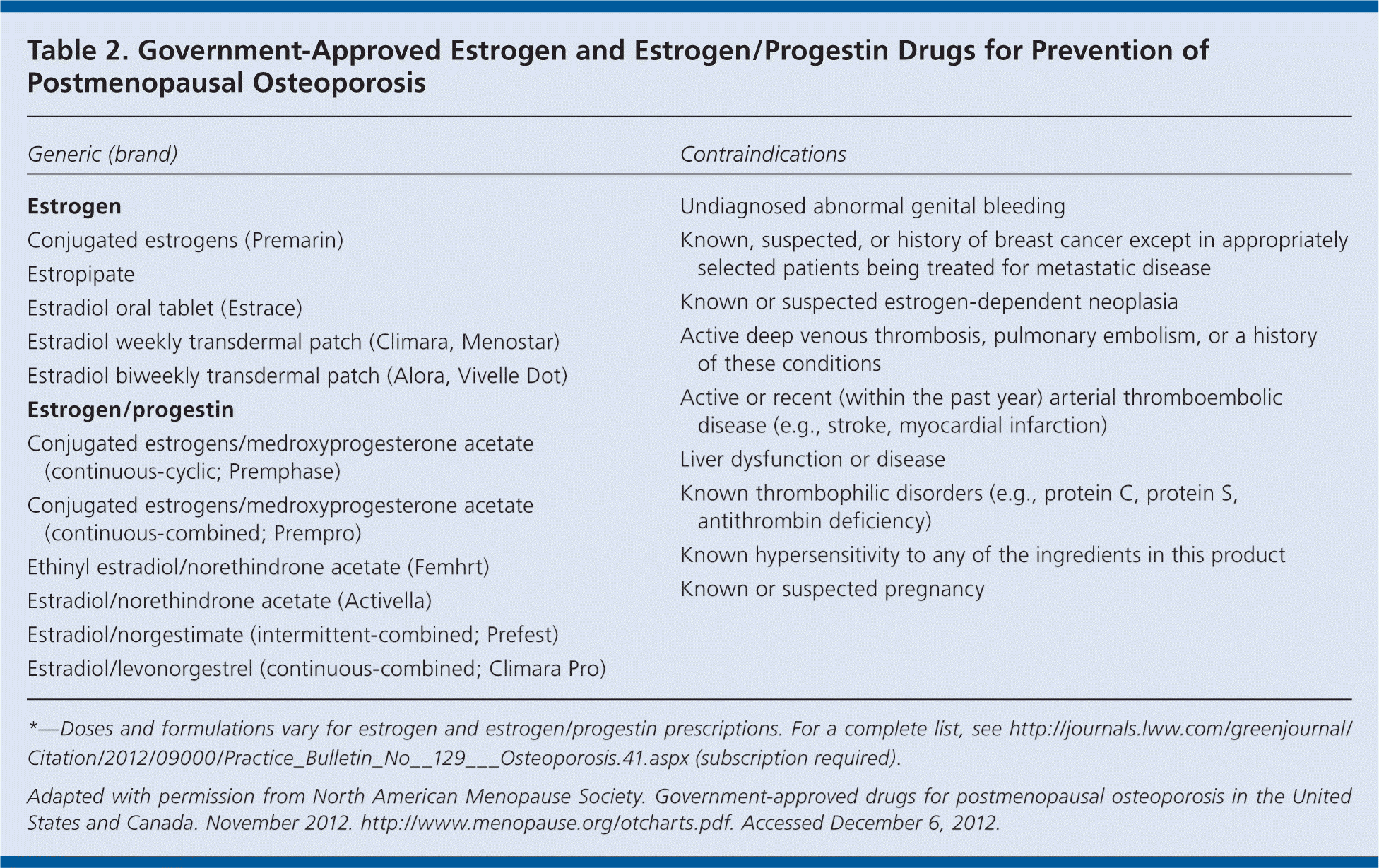
| Generic (brand) | Contraindications |
|---|---|
| Estrogen |
|
| Conjugated estrogens (Premarin) | |
| Estropipate | |
| Estradiol oral tablet (Estrace) | |
| Estradiol weekly transdermal patch (Climara, Menostar) | |
| Estradiol biweekly transdermal patch (Alora, Vivelle Dot) | |
| Estrogen/progestin | |
| Conjugated estrogens/medroxyprogesterone acetate (continuous-cyclic; Premphase) | |
| Conjugated estrogens/medroxyprogesterone acetate (continuous-combined; Prempro) | |
| Ethinyl estradiol/norethindrone acetate (Femhrt) | |
| Estradiol/norethindrone acetate (Activella) | |
| Estradiol/norgestimate (intermittent-combined; Prefest) | |
| Estradiol/levonorgestrel (continuous-combined; Climara Pro) |
If a patient loses bone mineral density during treatment, the physician should first determine if the patient is taking the medication correctly. The next appropriate step would be to assess the patient for secondary causes of osteoporosis, or to refer the patient to a specialist. The evaluation for secondary causes of osteoporosis includes two tiers of testing, with a complete blood count, metabolic profile, and 24-hour urine calcium, 25-hydroxyvitamin D, and thyroid-stimulating hormone measurement performed first, and a celiac panel and serum protein electrophoresis performed second.
