
Am Fam Physician. 2013;88(10):634-636
Author disclosure: No relevant financial affiliations.
TO THE EDITOR: Uterine perforation is a rare but potentially serious complication of the levonorgestrel-releasing intrauterine device (IUD; Mirena); the incidence is estimated at 0 to 2.6 per 1,000 insertions.1 Perforation typically occurs during IUD insertion, and symptoms can include abdominal pain and uterine bleeding. However, perforation can go unrecognized for months or years if asymptomatic.1–3
When recognized, an IUD that has perforated the uterus should be removed promptly because bowel perforation, obstruction, or adhesions can occur.1,2 Also, a malpositioned IUD may not prevent an unintended pregnancy.1–5 Although the levonorgestrel-releasing IUD can be inserted in a nonpregnant woman at any time, including immediately postpartum, the risk of perforation is greatest during the 12 weeks after giving birth and while the patient is lactating.1,4–6
We present the following case:
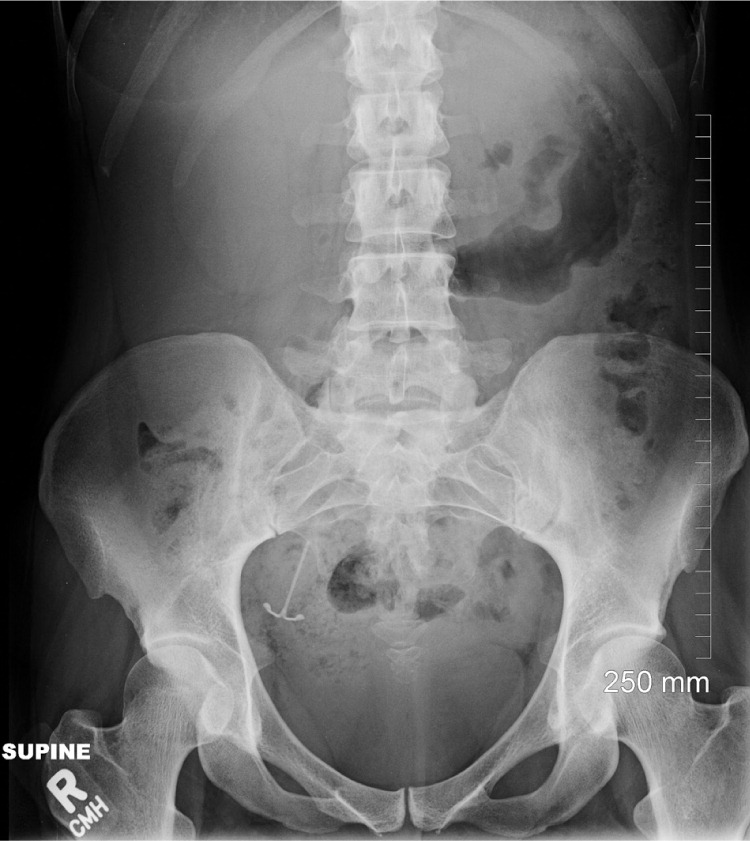
A 31-year-old woman (two pregnancies, one full-term delivery, one miscarriage, one living child) presented to a family medicine clinic 12 months after insertion of a levonorgestrel-releasing IUD with frequent, irregular, and increasingly heavy menstrual bleeding. She requested that the IUD be removed. It had been placed six weeks postpartum, and the patient was breastfeeding. Attempts to remove the IUD in the clinic were unsuccessful, and the use of abdominal and pelvic ultrasonography failed to locate it. Abdominal radiography revealed that the IUD was located in the right lateral pelvis (Figure 1). Although it was loosely tangled in the right fimbria, it was successfully removed using laparoscopy. No uterine structural abnormalities were found.
Clinicians and patients should carefully weigh the benefits and risks of IUD insertion during the postpartum period. A follow-up examination four to 12 weeks after insertion is recommended to ensure correct positioning; patients may opt to use another effective family planning method until this examination has occurred.
