
Am Fam Physician. 2013;88(11):776-777
Guideline source: American College of Obstetricians and Gynecologists
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Not reported
Published source: Obstetrics & Gynecology, November 2012
Available at: http://journals.lww.com/greenjournal/Abstract/2012/11000/Practice_Bulletin_No__131Screening_for_Cervical.49.aspx (subscription required)
The incidence of cervical cancer, as well as mortality rates from the disease, has decreased over the past 30 years because of widespread screening with cervical cytology. Screening technologies and risk-benefit considerations for different age groups continue to evolve. The American College of Obstetricians and Gynecologists (ACOG) has released a guideline on screening for cervical cancer.
Screening
INITIATION
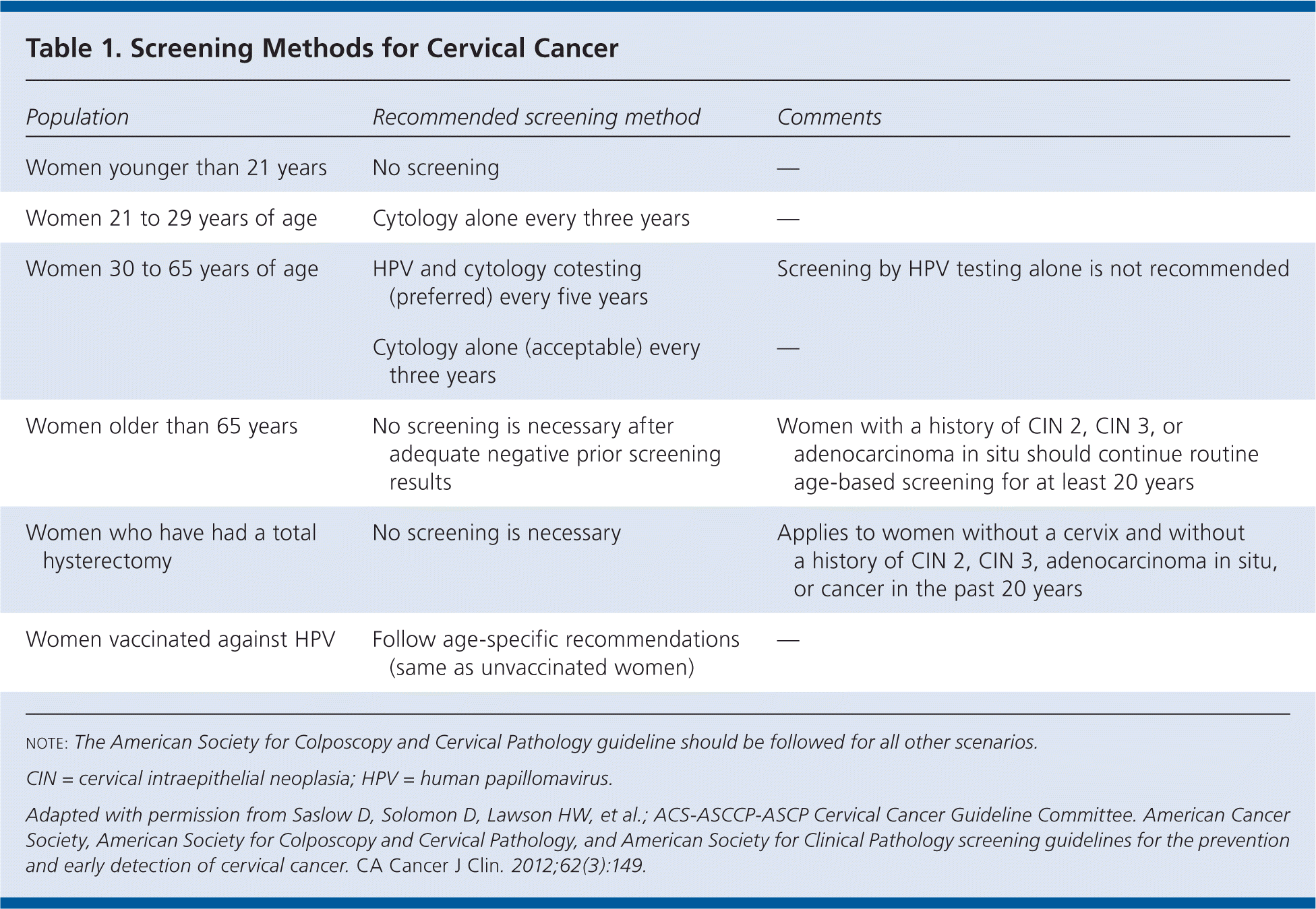
Screening should begin at 21 years of age, regardless of age at sexual initiation or other behavior-related risk factors. Table 1 summarizes screening methods and frequency. Human papillomavirus (HPV) vaccination does not affect screening recommendations.
| Population | Recommended screening method | Comments |
|---|---|---|
| Women younger than 21 years | No screening | — |
| Women 21 to 29 years of age | Cytology alone every three years | — |
| Women 30 to 65 years of age | HPV and cytology cotesting (preferred) every five years | Screening by HPV testing alone is not recommended |
| Cytology alone (acceptable) every three years | — | |
| Women older than 65 years | No screening is necessary after adequate negative prior screening results | Women with a history of CIN 2, CIN 3, or adenocarcinoma in situ should continue routine age-based screening for at least 20 years |
| Women who have had a total hysterectomy | No screening is necessary | Applies to women without a cervix and without a history of CIN 2, CIN 3, adenocarcinoma in situ, or cancer in the past 20 years |
| Women vaccinated against HPV | Follow age-specific recommendations (same as unvaccinated women) | — |
The incidence of cancer is low in women younger than 21 years, and there is a lack of evidence that screening is effective in this age group. Initiating screening before 21 years of age can increase anxiety, morbidity, expense, and unnecessary follow-up. Strategies for preventing cervical cancer in females younger than 21 years include HPV vaccination and counseling about safe sex practices.
WOMEN 21 TO 29 YEARS OF AGE
Cervical cytology should be performed every three years in women 21 to 29 years of age. Both liquid-based and conventional methods are acceptable. HPV testing is more sensitive but less specific than cytology. There is a high prevalence of high-risk HPV infections and a low incidence of cervical cancer in sexually active women younger than 30 years. Therefore, cotesting in this age group would mainly detect transient HPV infection without carcinogenic potential, leading to more testing without an appreciable decrease in cancer incidence.
Screening every three years in women 21 to 29 years of age requires less additional testing with similar reductions in cancer risk as screening every two years. Annual screening has a very small effect on cancer prevention and leads to excessive procedures and treatments.
WOMEN 30 TO 65 YEARS OF AGE
Use of both cytology and HPV testing every five years is preferred for healthy women 30 to 65 years of age, although cytology alone every three years is acceptable. Both liquid-based and conventional methods of cervical cytology are acceptable. Although increased sensitivity of cotesting allows for greater detection, decreased specificity leads to more follow-up testing. Performing cotesting every five years achieves slightly lower cancer rates with less screening and follow-up testing. Decision analyses show that cotesting every five years or cytology alone every three years provides a reasonable balance between the benefits and harms of screening.
WOMEN OLDER THAN 65 YEARS
Because cervical cancer usually occurs 15 to 25 years after HPV infection, screening women older than 65 years would prevent few cases of cancer. Screening should be discontinued at 65 years of age in women with a history of adequate negative screening results (i.e., three consecutive negative cytology results or two consecutive negative cotest results within the previous 10 years, with the most recent test performed within the past five years) and no history of cervical intraepithelial neoplasia (CIN) grade 2 or higher.
WOMEN WITH RISK FACTORS
Women with human immunodeficiency virus infection should be screened with cytology twice in the year after diagnosis, even if younger than 21 years, and annually thereafter. There are no studies regarding screening in women who are otherwise immunocompromised; however, annual cytology starting at 21 years of age is reasonable. Because women who have been treated for CIN 2 or higher have nearly a threefold increased risk of invasive disease for 20 years after treatment, they should receive annual, age-based screening during the 20 years after treatment or spontaneous regression, even if they reach 65 years of age.
WOMEN WHO HAVE HAD A TOTAL HYSTERECTOMY
Routine screening should be discontinued and not restarted for any reason in women who have had a hysterectomy with removal of the cervix and who have no history of CIN 2 or higher. The risk of developing vaginal cancer in this group is low, and continued screening is not effective.
Management
CYTOLOGY ALONE
Patients screened with cytology alone who have negative results should receive cytology screening again in three years. Those who have cytology results showing atypical squamous cells of undetermined significance should receive HPV testing. Patients with cytology results showing atypical squamous cells of undetermined significance and negative HPV results have low risk of CIN 3 and should be rescreened in three years. The American Society for Colposcopy and Cervical Pathology (ASCCP) guideline should be used for other scenarios.
COTESTING
Patients 30 years and older who receive negative results with cotesting should receive cotesting again in five years. A challenge with cotesting is the counseling and treatment of women 30 years and older with negative results on cytology but positive results on HPV testing. The risk of significant pathology is low in this group, and there are two management choices. The first option is to repeat cotesting in 12 months. If the cytology result shows low-grade squamous intraepithelial lesions or higher, or the HPV test result is still positive, the patient should be referred for colposcopy. Otherwise, the patient should receive routine screening. The second option is immediate HPV genotype-specific testing for HPV 16 or HPV 16/18. If the results of either test are positive, the patient should be referred for colposcopy. If the results for either test are negative, the patient should be cotested in 12 months. The ASCCP guideline should be used for other scenarios.
