
Am Fam Physician. 2013;88(12):821-822
Author disclosure: No relevant financial affiliations.
Ticagrelor (Brilinta) is a newer, reversible P2Y12 platelet inhibitor. Older, irreversible P2Y12 receptor inhibitors include prasugrel (Effient), clopidogrel (Plavix), and ticlopidine. Ticagrelor is labeled for the prevention of vascular events and death in patients with acute coronary syndrome.1
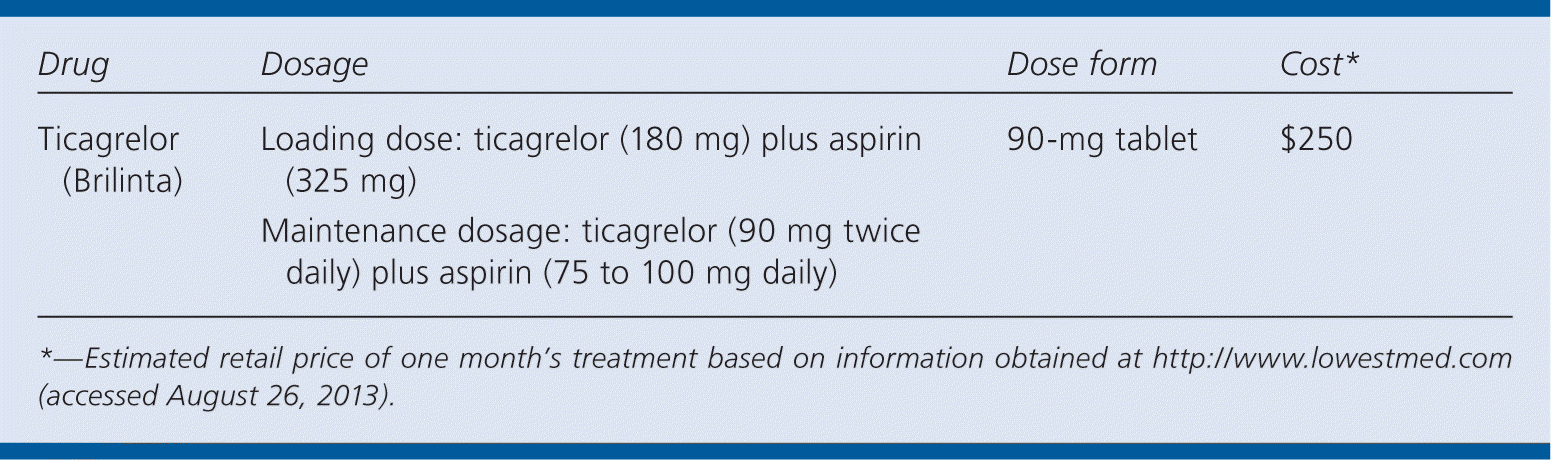
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Ticagrelor (Brilinta) | Loading dose: ticagrelor (180 mg) plus aspirin (325 mg) | 90-mg tablet | $250 |
| Maintenance dosage: ticagrelor (90 mg twice daily) plus aspirin (75 to 100 mg daily) |
SAFETY
The most significant safety issue with ticagrelor is the risk of bleeding episodes. A major bleeding episode will occur in 11.6% of patients, and life-threatening bleeding will affect 5.8% of patients over 12 months of therapy; these rates are similar to those for clopidogrel.1 Death caused by intracranial bleeding is more likely with ticagrelor than with clopidogrel (0.1% vs. 0.01%), but the likelihood of death caused by nonintracranial bleeding is lower (0.1% vs. 0.3%). Ticagrelor should not be used if patients are also taking cytochrome P450 3A4 inducers (e.g., rifampin, carbamazepine [Tegretol], dexamethasone, phenobarbital, phenytoin [Dilantin]) and strong CYP3A4 inhibitors (e.g., ketoconazole, ritonavir [Norvir], nefazodone). Ticagrelor increases bleeding risk in patients taking anticoagulants and antithrombotics. If these medications are used together, patients should be carefully monitored.2
Ticagrelor should not be used in patients with active pathologic bleeding (e.g., peptic ulcer), a history of intracranial bleeding when urgent coronary artery bypass graft surgery is anticipated, or within five days before any surgery. There is no reversal agent for ticagrelor, and it is not expected to be dialyzable. Ticagrelor is metabolized by the liver; use in patients with moderate to severe hepatic impairment could lead to an increased risk of bleeding or other complications. The manufacturer of ticagrelor recommends against using this drug in patients with severe hepatic impairment. Ticagrelor has not been studied in children or in women who are pregnant or breastfeeding. It is a U.S. Food and Drug Administration pregnancy category C drug.2
TOLERABILITY
Fewer than one in 10 patients will discontinue ticagrelor because of unwanted symptoms, a rate similar to that reported with clopidogrel. Patients taking ticagrelor report dyspnea about twice as often as patients taking clopidogrel (13.8% vs. 7.8%; number needed to harm = 17).1
EFFECTIVENESS
Ticagrelor is recommended for combination therapy with aspirin in patients who have acute coronary syndrome (unstable angina, non–ST elevation myocardial infarction, or ST elevation myocardial infarction) to reduce death from cardiovascular causes. Based on the results of a single international study of 18,624 patients from 43 countries, ticagrelor is more effective than clopidogrel in the combined outcome of preventing death from vascular causes, myocardial infarction, and stroke over the course of one year (9.8% vs. 11.7%; number needed to treat = 60; 95% confidence interval, 40 to 126).1 All-cause mortality was also decreased (4.5% vs. 5.9%; P < .001; number needed to treat = 70).1
Ticagrelor reduces myocardial infarction and vascular causes of death. However, this benefit was not demonstrated in North American study participants. It is hypothesized that this is because of the higher doses of aspirin used by this subgroup, but conclusive evidence is lacking.2,3 Ticagrelor does not have a beneficial effect on stroke-related death. A guideline from the American College of Cardiology and the American Heart Association recommends clopidogrel, ticagrelor, or prasugrel, in combination with aspirin, for dual-antiplatelet therapy for acute coronary syndrome.4 There are no head-to-head randomized trials comparing ticagrelor with prasugrel.
PRICE
A one-month supply of ticagrelor (two 90-mg tablets per day) costs approximately $250. This is significantly more expensive than generically available clopidogrel (two 75-mg tablets per day; approximately $23 for a one-month supply).5
SIMPLICITY
Ticagrelor must be taken twice daily, compared with the once-daily dosing of clopidogrel. A one-time loading dose of ticagrelor (180 mg) plus aspirin (325 mg) is followed by a maintenance dosage of ticagrelor (90 mg, taken twice daily) plus aspirin (75 to 100 mg daily) for 12 months. Doses of aspirin above 100 mg after the loading dose should not be used.2
Bottom Line
Ticagrelor is an alternative to clopidogrel for secondary prevention of cardiovascular death in patients with acute coronary syndrome. In an international study it was found to be more effective, but patients in the United States did not experience superior outcomes. Overall, ticagrelor is more expensive, more often causes adverse effects, and has a less convenient dosing schedule. For most patients in the United States, clopidogrel will likely be a better choice.
