Am Fam Physician. 2014;89(4):265-272
Related U.S. Preventive Services Task Force Recommendation Statement: Screening for HIV
Related Putting Prevention into Practice: Screening for HIV
Related editorial: AAFP Recommends Universal Screening for HIV Infection Beginning at 18 Years of Age
Author disclosure: No relevant financial affiliations.
Human immunodeficiency virus (HIV) prevention and treatment updates include screening recommendations, fourth-generation testing, preexposure prophylaxis, and a paradigm shift; treatment is prevention. The U.S. Preventive Services Task Force recommends routine HIV screening in persons 15 to 65 years of age, regardless of risk. Fourth-generation testing is replacing the Western blot and can identify those with acute HIV infection. The U.S. Food and Drug Administration approved the OraQuick In-Home HIV Test; however, there are concerns about reduced sensitivity, possible misinterpretation of results, potential for less effective counseling, and possible cost barriers. Preexposure prophylaxis (effective in select high-risk adult populations) is the combination of safer sex practices and continuous primary care prevention services, plus combination antiretroviral therapy. Concerns for preexposure prophylaxis include the necessity of strict medication adherence, limited use among high-risk populations, and community misconceptions of appropriate use. Evidence supports combination antiretroviral therapy as prevention for acute HIV infection, thus lowering community viral loads. Evidence has increased supporting combination antiretroviral therapy for treatment at any CD4 cell count. Resistance testing should guide therapy in all patients on entry into care. Within two weeks of diagnosis of most opportunistic infections, combination antiretroviral therapy should be started; patients with tuberculosis and cryptococcal meningitis require special considerations.
Persistent high rates of human immunodeficiency virus (HIV) transmission in the United States require new strategies to combat the ongoing epidemic. Latest recommendations and evidence address routine HIV screening, implementation of fourth-generation testing, targeted use of preexposure prophylaxis (PrEP) for high-risk adults without HIV, treatment as prevention to lower community viral loads, and treatment guidelines on single fixed-dose combination antiretroviral therapy (CART).1–7
| Clinical recommendation | Evidence rating | Reference |
|---|---|---|
| All adolescents and adults 15 to 65 years of age should be screened for HIV unless they explicitly refuse. | A | 1 |
| All persons at high risk younger than 15 years and older than 65 years should be screened for HIV. | A | 1 |
| Rapid HIV tests should be used for screening, including fourth-generation testing when available. | C | 1, 9, 10 |
| All pregnant women should be screened for HIV during each pregnancy. | A | 1 |
| Preexposure prophylaxis should be provided to men and women (except those who are breastfeeding) who are at highest risk of HIV infection (e.g., men who have sex with men, those with an HIV-positive sex partner). | A | 2–4 |
| It is recommended that combination antiretroviral therapy be initiated early to prevent HIV transmission. | A | 1, 5, 7 |
What Is New in Screening?
The U.S. Preventive Services Task Force recommends routine HIV screening, known as opt-out screening, regardless of patient or physician perception of risk for all persons 15 to 65 years of age, unless a patient refuses.1 Those younger than 15 years and older than 65 years with risk factors should also be screened, including men who have sex with men; injection drug users; persons having unprotected vaginal or anal intercourse; persons who have a sex partner with high-risk behaviors; persons with a history of or current concern for other sexually transmitted infections; and sex workers.1 The Centers for Disease Control and Prevention recommends routine HIV testing for all persons 13 to 64 years of age.8 Although little evidence exists to determine the interval for rescreening, at least annual HIV screening is recommended for groups at high risk. Less frequent screening intervals are appropriate for other groups, including those younger than 15 years and older than 65 years.1 Use of rapid tests in screening programs is supported by the evidence, because they have been found to be highly accurate; however, subsequent conventional testing is necessary to confirm an HIV diagnosis.1,9,10
The U.S. Preventive Services Task Force recommends HIV screening in all pregnant women (even if in active labor), despite previous pregnancy HIV status.1 The Centers for Disease Control and Prevention recommends HIV screening at entry into care and repeat screening in the third trimester in women living in areas with high HIV rates among pregnant women.8 If HIV infection is detected, effective care and partner notification are essential for disease control.
WHAT ARE THE ADVANTAGES OF OPT-OUT SCREENING?
IS THERE ANY HARM FROM AT-RISK OPT-IN SCREENING?
Opt-in screening based on demographic, behavioral, or clinical subpopulations is known to lower test rates, but identifies only 75% of patients with HIV,13 resulting in a large number of persons presenting late in disease progression.14 Other limitations include patient perception of risk, reluctance for self-disclosure, and lack of comprehensive risk assessments.
What Is New in Testing?
Serologic testing is enhanced by a newer fourth-generation algorithm allowing for identification of early HIV infection.15,16 Additionally, the U.S. Food and Drug Administration (FDA) licensed the OraQuick In-Home HIV Test.17 Earlier generation rapid HIV tests should be used for screening in areas where blood collection is not feasible; later generation blood-based rapid fourth-generation testing is preferred for screening when available.
WHAT IS FOURTH-GENERATION TESTING?
The fourth-generation testing uses combined antibody/antigen immunoassay (as opposed to antibodies alone) to identify HIV-1 and HIV-2.18 If the results on the primary antibody/antigen assay are positive, another immunoassay differentiates between HIV-1 and HIV-2 antibodies and helps guide the choice of CART. HIV-2, which is less common than HIV-1 and found mostly in West Africa, is resistant to non-nucleoside reverse transcriptase inhibitors and enfuvirtide (Fuzeon) therapy.7,19 If the results of the second-tier HIV-1/HIV-2 immunoassay are negative, a nucleic acid amplification test is performed to detect HIV RNA viral activity rather than antibodies to HIV.18 A positive result on nucleic acid amplification testing identifies acute HIV-1 infection (rare false-positive results are possible).
Third-generation assays consist of rapid enzyme immunoassays that look for antibodies or parts of antibodies on the surface of HIV, and one or more confirmatory tests, such as the Western blot. These assays can detect immunoglobulin M antibodies, which increase first, and immunoglobulin G antibodies, which increase later. The less-sensitive Western blot is the confirmatory test that looks for HIV antibodies, which may take up to three months to confirm, with results ranging from positive or negative to inconclusive.20
HOW MUCH EARLIER CAN A DIAGNOSIS BE MADE WITH FOURTH-GENERATION TESTING?
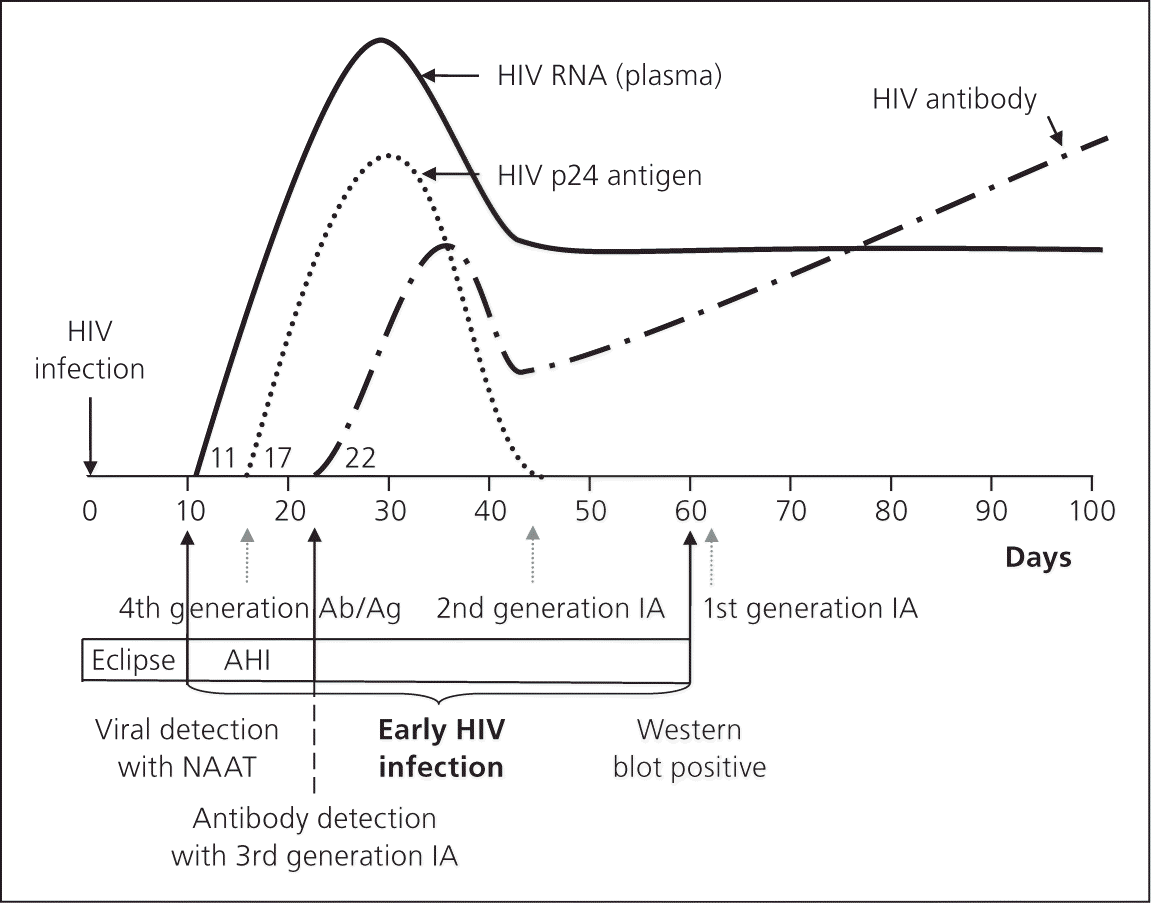
The window period is from initial HIV infection until any laboratory test detects HIV. Fourth-generation testing incorporates HIV-1/HIV-2 antibody and p24 antigen detection; therefore, the window period can be as early as 15 to 17 days (Figure 1).16 Using fourth-generation testing, HIV-1 infection can be diagnosed within two to three weeks of risk exposure.16 Qualitative HIV-1 nucleic acid amplification tests and HIV-1 viral load tests are positive around day 10 to 12; however, these tests are not typically used for screening purposes. A rapid finger stick blood-based test (Alere Determine) that detects and differentiates HIV-1/HIV-2 antibodies and HIV-1 p24 antigen has recently been approved by the FDA.21
WHAT IS THE ADVANTAGE OF FOURTH-GENERATION TESTING?
Fourth-generation testing can screen for and confirm HIV infection in several hours instead of weeks, allows for identification of very early HIV infection, and eliminates the indeterminate Western blot results that could take up to three to six months to confirm.15 Persons at high risk can be retested within three weeks of potential exposure. Identification of acute HIV infection allows for prevention of new infections through risk reduction counseling, earlier intervention with CART, and partner notification.7 Fourth-generation testing costs more per sample than previous algorithms; however, preliminary cost-effectiveness studies on fourth-generation testing in high-incidence areas found benefits of increased case identification, reduced transmission, and increased quality-adjusted life years.22
WHAT IS THE NEW OVER-THE-COUNTER TEST?
The FDA has approved the OraQuick In-Home HIV Test, which is a rapid test that does not require sample shipment for laboratory confirmation.17 The test, which is available to persons older than 17 years, provides results in 20 to 40 minutes. Positive results require confirmation with a Western blot or fourth-generation testing. A limitation is that it has a sensitivity of 92% when the test is self-administered because of user error and poor technique, compared with a sensitivity of 99% when administered by trained professionals.2 Other limitations are misinterpretation of tests during the window period, potential for less effective counseling, and possible cost barriers.
What Is PrEP?
PrEP for sexual transmission of HIV is the combination of continual delivery of counseling on behavioral risk reduction, techniques for medication adherence, easy access to condoms, monitoring of pregnancy status, sexually transmitted infection screening and treatment, and strict adherence to once-daily oral combination therapy of 200-mg emtricitabine/300-mg tenofovir (Truvada).2,3 Studies support PrEP effectiveness in reducing HIV infections in high-risk settings. Concern exists for development of resistance in settings of nonadherence.23 A consistent observation among PrEP studies of men who have sex with men and of men who have sex with women is remarkably poor adherence to drug regimens, as measured by drug levels.24,25
WHO CAN RECEIVE PrEP BASED ON CLINICAL EVIDENCE?
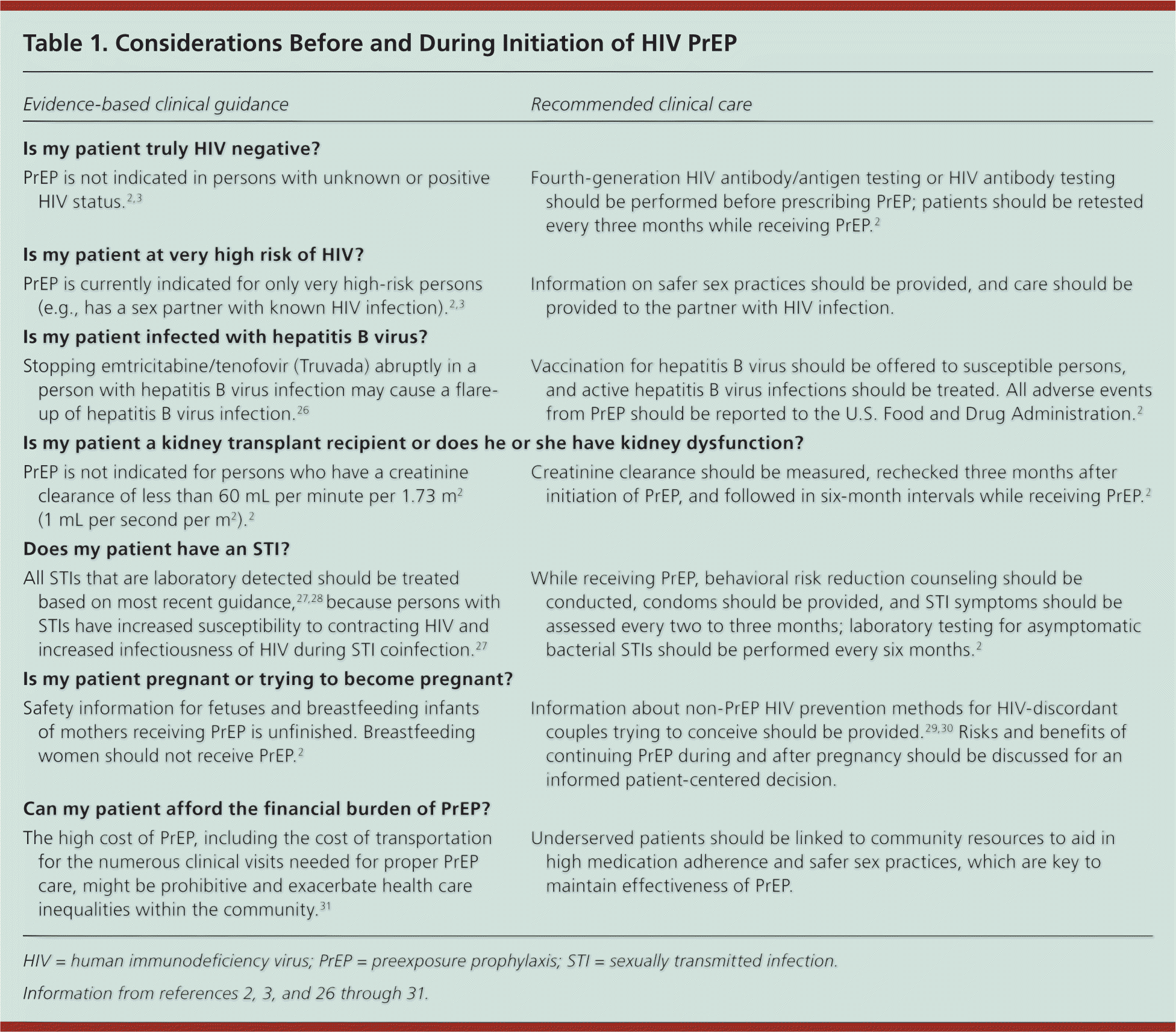
Only HIV-negative persons are candidates for PrEP as a part of a prevention strategy that includes ongoing risk reduction and condom counseling. Assessment for HIV before and routinely during enrollment is required. PrEP is recommended for adults (i.e., persons older than 18 years) at very high risk (e.g., has a sex partner with known HIV infection), who have negative results on a fourth-generation HIV antibody/antigen test or HIV antibody test at the time of PrEP enrollment, and who agree to repeat HIV testing every three months2–4,24,25 (Table 12,3,26–31 ). Figure 2 outlines a process to identify potential PrEP candidates.2,3 Guidance for the use of PrEP can be found at http://www.cdc.gov/hiv/prevention/research/prep/index.html and http://www.truvadapreprems.com.
| Evidence-based clinical guidance | Recommended clinical care |
|---|---|
| Is my patient truly HIV negative? | |
| PrEP is not indicated in persons with unknown or positive HIV status.2,3 | Fourth-generation HIV antibody/antigen testing or HIV antibody testing should be performed before prescribing PrEP; patients should be retested every three months while receiving PrEP.2 |
| Is my patient at very high risk of HIV? | |
| PrEP is currently indicated for only very high-risk persons (e.g., has a sex partner with known HIV infection).2,3 | Information on safer sex practices should be provided, and care should be provided to the partner with HIV infection. |
| Is my patient infected with hepatitis B virus? | |
| Stopping emtricitabine/tenofovir (Truvada) abruptly in a person with hepatitis B virus infection may cause a flare-up of hepatitis B virus infection.26 | Vaccination for hepatitis B virus should be offered to susceptible persons, and active hepatitis B virus infections should be treated. All adverse events from PrEP should be reported to the U.S. Food and Drug Administration.2 |
| Is my patient a kidney transplant recipient or does he or she have kidney dysfunction? | |
| PrEP is not indicated for persons who have a creatinine clearance of less than 60 mL per minute per 1.73 m2 (1 mL per second per m2).2 | Creatinine clearance should be measured, rechecked three months after initiation of PrEP, and followed in six-month intervals while receiving PrEP.2 |
| Does my patient have an STI? | |
| All STIs that are laboratory detected should be treated based on most recent guidance,27,28 because persons with STIs have increased susceptibility to contracting HIV and increased infectiousness of HIV during STI coinfection.27 | While receiving PrEP, behavioral risk reduction counseling should be conducted, condoms should be provided, and STI symptoms should be assessed every two to three months; laboratory testing for asymptomatic bacterial STIs should be performed every six months.2 |
| Is my patient pregnant or trying to become pregnant? | |
| Safety information for fetuses and breastfeeding infants of mothers receiving PrEP is unfinished. Breastfeeding women should not receive PrEP.2 | Information about non-PrEP HIV prevention methods for HIV-discordant couples trying to conceive should be provided.29,30 Risks and benefits of continuing PrEP during and after pregnancy should be discussed for an informed patient-centered decision. |
| Can my patient afford the financial burden of PrEP? | |
| The high cost of PrEP, including the cost of transportation for the numerous clinical visits needed for proper PrEP care, might be prohibitive and exacerbate health care inequalities within the community.31 | Underserved patients should be linked to community resources to aid in high medication adherence and safer sex practices, which are key to maintain effectiveness of PrEP. |

IS PrEP SAFE FOR WOMEN TRYING TO CONCEIVE, AND PREGNANT OR BREASTFEEDING WOMEN?
Safety information for fetuses and breastfeeding infants of HIV-discordant couples using PrEP is unfinished; no adverse events for fetuses and breastfeeding infants exposed to emtricitabine/tenofovir have been reported. Non-PrEP HIV prevention options are available for HIV-discordant couples trying to conceive.27,29 The Centers for Disease Control and Prevention recommends against prescribing PrEP to breastfeeding women (information submitted to the FDA's MedWatch) if PrEP is continued during a pregnancy.2 It should be noted that nonadherence to PrEP in clinical trials, as evidenced by drug levels or pill counts, was associated with failure to prevent HIV infection.32 A key study of women was terminated because of lack of efficacy.31
WHAT DO I NEED TO EMPHASIZE TO MY PATIENT RECEIVING PrEP?
It is important that patients receiving PrEP adhere to follow-up appointments and take medication as prescribed; this is not a “just before sex pill.” PrEP effectiveness depends on medication adherence and safer sex practices. Among men who have sex with men, HIV risk reduction was 73% with at least 90% medication adherence. Adherence less than 90% reduced risk by 21%.33 Physicians should inform patients about unknown long-term effects of medication, inquire about medication adherence and adverse effects (e.g., diarrhea, dizziness, nausea), reinforce adherence to behavior changes,1 and provide specific evidence-based HIV behavioral interventions.34,35
What Is New in Prevention and Treatment?
TREATMENT AS PREVENTION
The most important evidence-based message is that early treatment is viewed as an integral part of community-based prevention.5,36 Patient adherence to CART, even for early HIV infection (CD4 cell count greater than 350 per mm3 [0.35 × 109 per L]), provides hope of lessening disease progression through control of HIV, and contributing to a lower community viral load and reduced risk of HIV transmission.37 Hence, early treatment is prevention. It may be that there is more community benefit vs. individual benefit with early CART. Ongoing clinical trials to improve understanding are needed.
WHAT IS NEW IN TREATMENT FOR CART-NAIVE PATIENTS?
Research shows CART is effective in lowering viral loads to nearly undetectable levels, advancing the concept that CART is prevention.5 Early antiretroviral therapy has shown clinical benefit when initiated at a CD4 cell count between 350 and 550 cells per mm3 (0.35 and 0.55 × 109 per L) by reducing HIV-related clinical events.5 A small pilot study of early CART, initiated within the first weeks of infection, resulted in normal CD8 cell activation 48 and 96 weeks after treatment began—comparable to healthy patients without HIV in the control group.38 Further research is needed to evaluate the potential for superior clinical outcomes.38
Patients willing to commit to early CART should understand risks of drug resistance if nonadherent, unknown long-term treatment toxicities,7,37 and ongoing controversies for early treatment.8,39 Also, the aging population with HIV infection and comorbidities increases concern for polypharmacy and drug-drug interactions. The latest clinical and prevention guidelines for HIV treatment are available at http://aidsinfo.nih.gov/guidelines. Consultation with an expert is advised when early (acute HIV infection) treatment is undertaken.
SHOULD CART BE INITIATED IN THE SETTING OF ACUTE OPPORTUNISTIC INFECTIONS?
Within the first two weeks of diagnosis of most opportunistic infections, CART should be started. Patients with tuberculosis and cryptococcal meningitis are exceptions; these patients may require delayed CART initiation to improve clinical outcomes. Treatment of tuberculosis in the setting of CART is further complicated by drug interactions. Once CART has been started in patients with tuberculosis or cryptococcal meningitis, close monitoring for and treatment of immune reconstitution inflammatory syndrome, which restores the immune response to the infection with secondary severe inflammatory consequences, is of critical importance. Expert consultation is recommended in these patients. Additional resources are available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf and http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
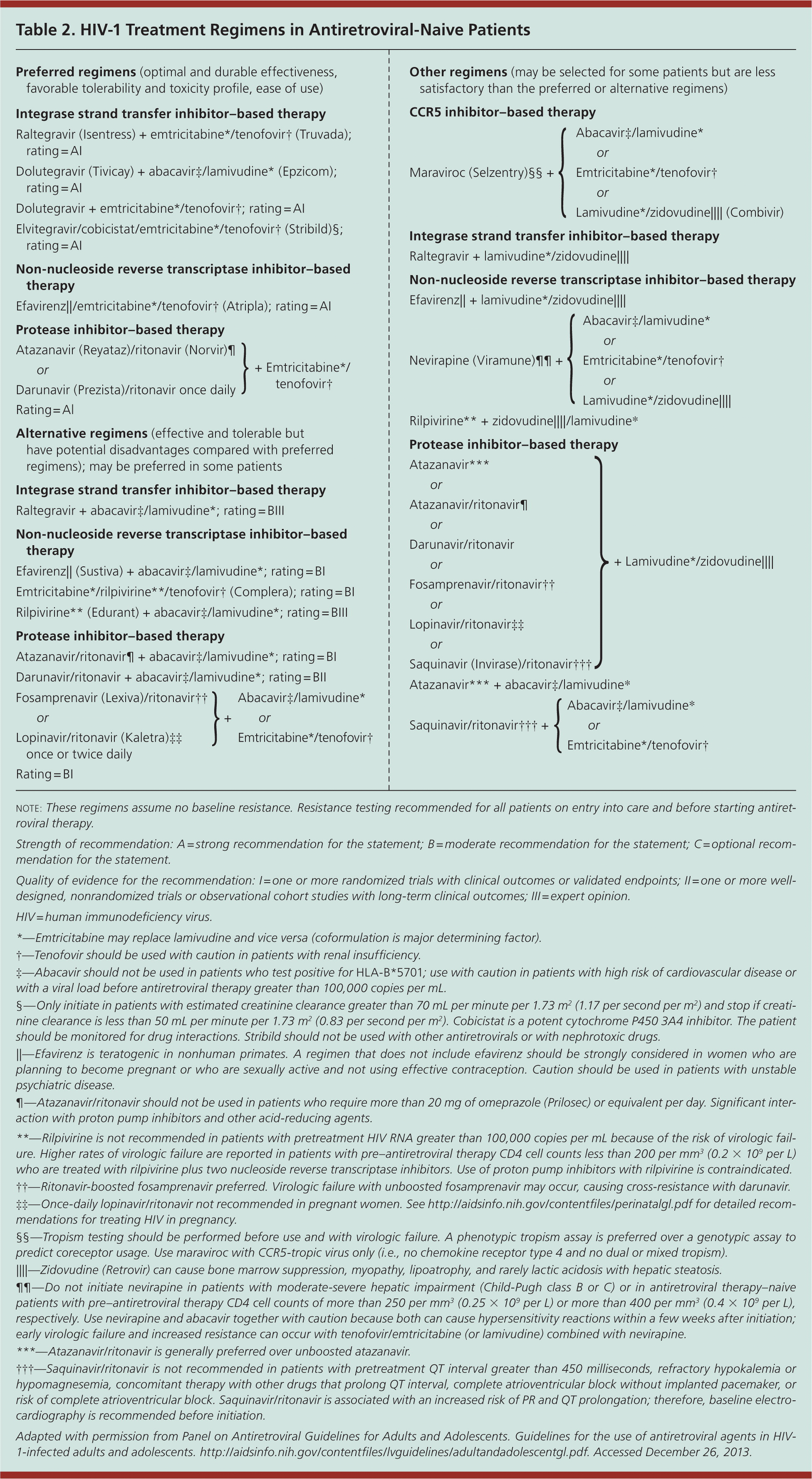
WHAT CART IS RECOMMENDED FOR INITIAL THERAPY?
Therapy should be guided by resistance testing in all patients on entry into care; however, resistance testing is inconsistently performed.7 Recommendations for CART include preferred combinations of nucleoside/nucleotide reverse transcriptase inhibitor–, non-nucleoside reverse transcriptase inhibitor–, ritonavir (Norvir)-boosted protease inhibitor–, or integrase strand transfer inhibitor–based regimens.7,37 Six classes of HIV medications are approved for initial therapy (Table 27 ). Guidance on CART for antiretroviral-naive patients is available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf and https://www.iasusa.org/content/antiretroviral-treatment-adult-hiv-infection-0.
WHAT IS NEW FOR CART?
The FDA has approved the one-pill combination of elvitegravir (an HIV-1 integrase strand transfer inhibitor)/cobicistat (a pharmacokinetic booster)/emtricitabine/tenofovir (Stribild) as an alternative CART regimen for patients with a creatinine clearance greater than 70 mL per minute per 1.73 m2 (1.17 per second per m2).7,37 Stribild was found to be equivalent to efavirenz (Sustiva)- and atazanavir (Reyataz)-based regimens. Stribild is well tolerated, but carries potential for drug interactions with its coformulated pharmacokinetic booster, cobicistat. Also, cobicistat, through inhibition of tubular secretion of creatinine, causes a mild increase in serum creatinine of about 0.1 to 1.5 mg per dL (9 to 133 μmol per L).40,41
Of the one pill per day combination CART therapies available, efavirenz/emtricitabine/tenofovir (Atripla) is a preferred regimen and rilpivirine/emtricitabine/tenofovir (Complera) and Stribild are alternative regimens approved for use in antiretroviral-naive patients. Dolutegravir (Tivicay) is an integrase strand transfer inhibitor approved by the FDA in August 2013 for treating HIV-1 in treatment-naive adults (including those with prior integrase strand transfer inhibitor exposure), and treatment-naive and treatment-experienced children 12 years and older (weighing at least 88 lb [40 kg]) who have never taken other integrase strand transfer inhibitor medications. Dosing depends on whether the patient is integrase strand transfer inhibitor–naive or experienced with integrase strand transfer inhibitors, on resistance at baseline, and on certain drug interactions. Prescribing information can be found at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf.
Data Sources: The literature review included PubMed, UpToDate, Google search, Clinical Evidence, NIH HIV/AIDS Treatment Guidelines, U.S. Preventive Services Task Force, Essential Evidence Plus, and Cochrane Database of Systematic Reviews. Keywords included HIV testing, acute HIV infection diagnosis, acute retroviral syndrome diagnosis, primary HIV infection diagnosis, 4th generation HIV testing, preexposure prophylaxis, HIV antiretroviral therapy, new HIV antiretroviral medications, investigational antiretroviral medications, elvitegravir, QUAD pill, and emerging HIV therapeutics. All searches were completed by October 30, 2013.