
Am Fam Physician. 2014;89(5):368-377
A more recent article on common sleep disorders in children is available.
Author disclosure: No relevant financial affiliations.
Up to 50% of children will experience a sleep problem. Early identification of sleep problems may prevent negative consequences, such as daytime sleepiness, irritability, behavioral problems, learning difficulties, motor vehicle crashes in teenagers, and poor academic performance. Obstructive sleep apnea occurs in 1% to 5% of children. Polysomnography is needed to diagnose the condition because it may not be detected through history and physical examination alone. Adenotonsillectomy is the primary treatment for most children with obstructive sleep apnea. Parasomnias are common in childhood; sleepwalking, sleep talking, confusional arousals, and sleep terrors tend to occur in the first half of the night, whereas nightmares are more common in the second half of the night. Only 4% of parasomnias will persist past adolescence; thus, the best management is parental reassurance and proper safety measures. Behavioral insomnia of childhood is common and is characterized by a learned inability to fall and/or stay asleep. Management begins with consistent implementation of good sleep hygiene practices, and, in some cases, use of extinction techniques may be appropriate. Delayed sleep phase disorder is most common in adolescence, presenting as difficulty falling asleep and awakening at socially acceptable times. Treatment involves good sleep hygiene and a consistent sleep-wake schedule, with nighttime melatonin and/or morning bright light therapy as needed. Diagnosing restless legs syndrome in children can be difficult; management focuses on trigger avoidance and treatment of iron deficiency, if present.
Sleep is one of the most commonly discussed topics during well-child visits.1 It is important for primary care physicians to be familiar with normal childhood sleep patterns and common sleep disorders. Epidemiologic studies indicate that up to 50% of children experience a sleep problem,2–4 and about 4% have a formal sleep disorder diagnosis.5
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Sleep disorders should be considered in children presenting with irritability, behavioral problems, learning difficulties, and poor academic performance. | C | 6–8, 52, 53 |
| Adenotonsillectomy is the primary treatment for children with obstructive sleep apnea. | B | 13, 56–58 |
| Sleep or sedating medications have no role in the treatment of behavioral insomnia of childhood. | C | 29, 30 |
| If restless legs syndrome is suspected in a child, management should include a workup for iron deficiency and avoidance of triggers. | C | 47–50 |
Normal Sleep in Infants and Children
Sleep is an opportunity for the body to conserve energy, restore its normal processes, promote physical growth, and support mental development. The most recognized consequence of inadequate sleep is daytime sleepiness. However, sleepiness in children commonly manifests as irritability, behavioral problems, learning difficulties, motor vehicle crashes in teenagers, and poor academic performance.6–8 Distinguishing significant sleep disruptions from normal age-related changes can be challenging and can ultimately delay treatment.
Sleep changes considerably during the first few years of life and parallels physical maturation and development. Newborns require the greatest total sleep time and have a fragmented sleep-wake pattern. Starting at five months of age, infants have the ability to sleep for longer periods. At six months of age, children are able to go without nighttime feedings, but significant variation exists. Additionally, breastfeeding infants have more frequent awakenings, shorter sleep periods, and slightly shorter total sleep times.9 As children age, sleep periods gradually lengthen and total sleep time decreases (Figure 1).
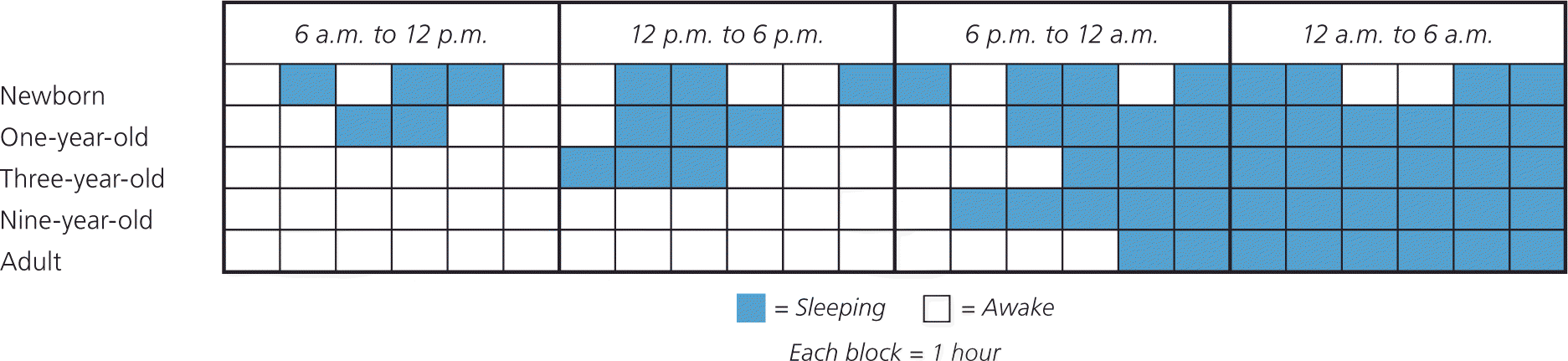
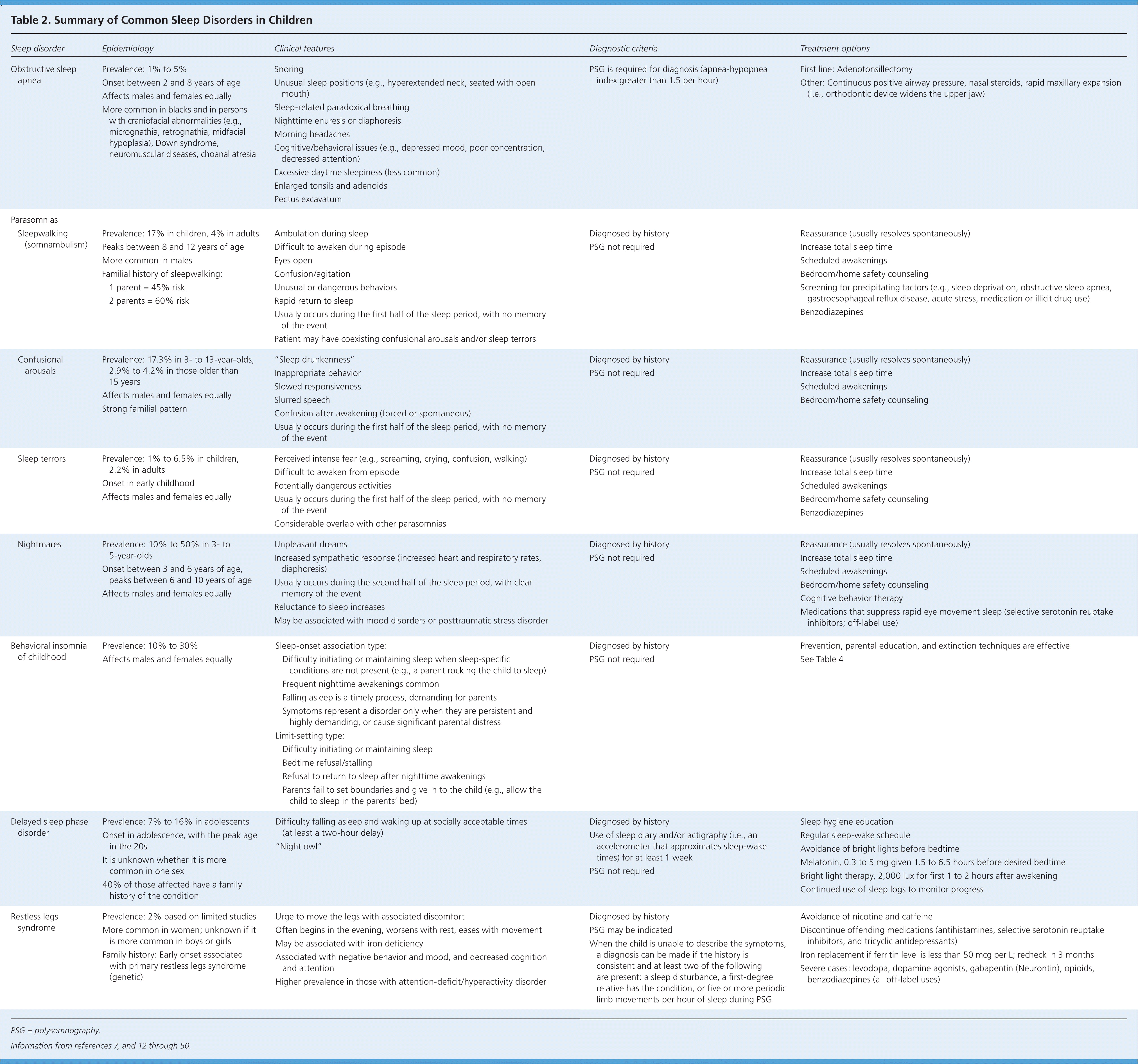
The large variation in sleep behavior among children may be secondary to cultural or genetic differences; however, there are some general trends (Table 1).10,11 Ultimately, knowing the normal developmental stages of sleep will help differentiate between normal sleep and common sleep disorders, such as obstructive sleep apnea (OSA), parasomnias, behavioral insomnia of childhood, delayed sleep phase disorder, and restless legs syndrome. These disorders are summarized in Table 2.7,12–50
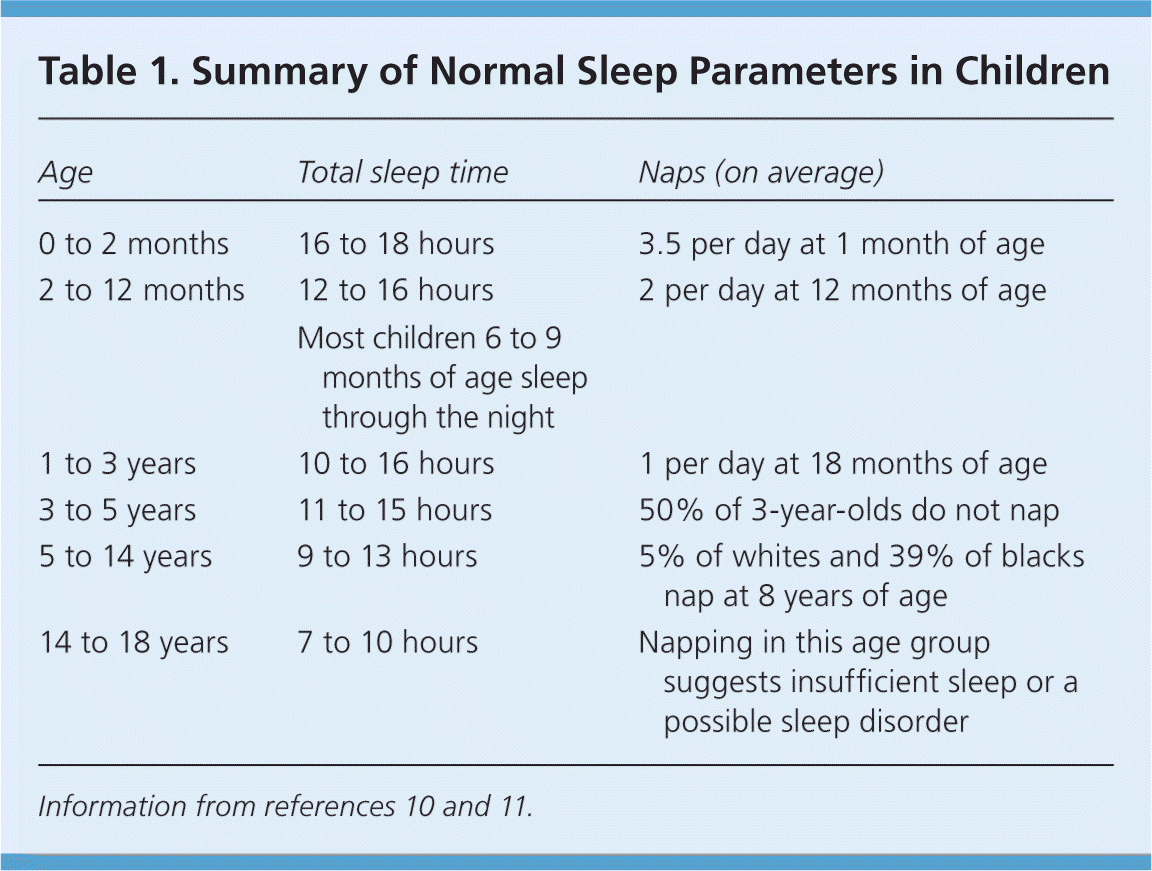
| Age | Total sleep time | Naps (on average) |
|---|---|---|
| 0 to 2 months | 16 to 18 hours | 3.5 per day at 1 month of age |
| 2 to 12 months | 12 to 16 hours | 2 per day at 12 months of age |
| Most children 6 to 9 months of age sleep through the night | ||
| 1 to 3 years | 10 to 16 hours | 1 per day at 18 months of age |
| 3 to 5 years | 11 to 15 hours | 50% of 3-year-olds do not nap |
| 5 to 14 years | 9 to 13 hours | 5% of whites and 39% of blacks nap at 8 years of age |
| 14 to 18 years | 7 to 10 hours | Napping in this age group suggests insufficient sleep or a possible sleep disorder |
| Sleep disorder | Epidemiology | Clinical features | Diagnostic criteria | Treatment options | |||
|---|---|---|---|---|---|---|---|
| Obstructive sleep apnea |
|
|
|
| |||
| Parasomnias | |||||||
| Sleepwalking (somnambulism) |
|
|
|
| |||
| Confusional arousals |
|
|
|
| |||
| Sleep terrors |
|
|
|
| |||
| Nightmares |
|
|
|
| |||
| Behavioral insomnia of childhood |
|
|
|
| |||
| Delayed sleep phase disorder |
|
|
|
| |||
| Restless legs syndrome |
|
|
|
| |||
Obstructive Sleep Apnea
OSA is characterized by upper airway obstruction, despite respiratory effort, that disrupts normal sleep patterns and ventilation.12 OSA can be associated with obesity, excessive soft tissue in the upper airway, decreased upper airway lumen size, or failure of pharyngeal dilator muscles. However, in children, the obstruction is primarily due to enlarged tonsils and adenoids.12,13 Onset usually occurs between two and eight years of age, coinciding with peak tonsil growth, but the condition can manifest at any age.51 The overall prevalence in children is 1% to 5%.13 It occurs equally among males and females, but is more common in ethnic minorities.13
Snoring and witnessed apneas are the classic symptoms of OSA, but not all snorers have the condition. The prevalence of habitual snoring in children is as high as 27%, which can complicate the recognition of OSA.13,16,52 Other common symptoms include unusual sleeping positions (e.g., hyperextended neck, seated with open mouth), sleep-related paradoxical breathing, nighttime diaphoresis or enuresis, morning headaches, and excessive daytime sleepiness. However, children are less likely than adults to present with daytime sleepiness. Sleepiness in children is more likely to manifest as depressed mood, poor concentration, decreased attention, or behavioral issues.6,52,53
Weight and body mass index are usually normal in children with OSA; however, the incidence of obesity-related sleep apnea is steadily increasing.5,54 Physical examination findings can include enlarged tonsils, micrognathia, and pectus excavatum. However, subjective grading of tonsil size in children does not always correlate with objective findings.55
Results of the history and physical examination alone correlate poorly with objective findings of OSA, and questionnaires have shown a sensitivity of only 78%.13 Therefore, children with suspected OSA should be referred for polysomnography.17–19 In addition, referral to a sleep medicine specialist should be considered for those with high-risk features (e.g., attention-deficit/hyperactivity disorder, cardiorespiratory failure, craniofacial abnormalities, congenital defects, Down syndrome).
Untreated OSA is associated with neurobehavioral problems, decreased attention, disturbed emotional regulation, decreased academic performance, nighttime enuresis, impaired growth, and, rarely, systemic hypertension, pulmonary hypertension, and cor pulmonale.6,17–20,52,53 Adenotonsillectomy is the primary treatment for OSA in children (Table 3).13,18 Following adenotonsillectomy, postoperative polysomnography demonstrates resolution of OSA in more than 70% of normal-weight children, but in less than one-half of obese children.13,56–58 Postoperative improvements in quality of life and behavior may also occur.56–59 Children being considered for adenotonsillectomy who are at high risk of postoperative complications (risk factors include age younger than three years, severe OSA, obesity, current respiratory infection, craniofacial abnormalities, failure to thrive, cardiac complications of OSA, and neuromuscular disorders) should undergo the procedure as an inpatient. Once treated, all children should have a clinical assessment six to eight weeks postoperatively, and polysomnography should be repeated to assess for residual OSA in those with obesity, moderate to severe OSA on initial testing, or persistent symptoms.13,17,18 If ordered, polysomnography should be performed after the pharynx has fully healed, usually no earlier than six weeks postoperatively.
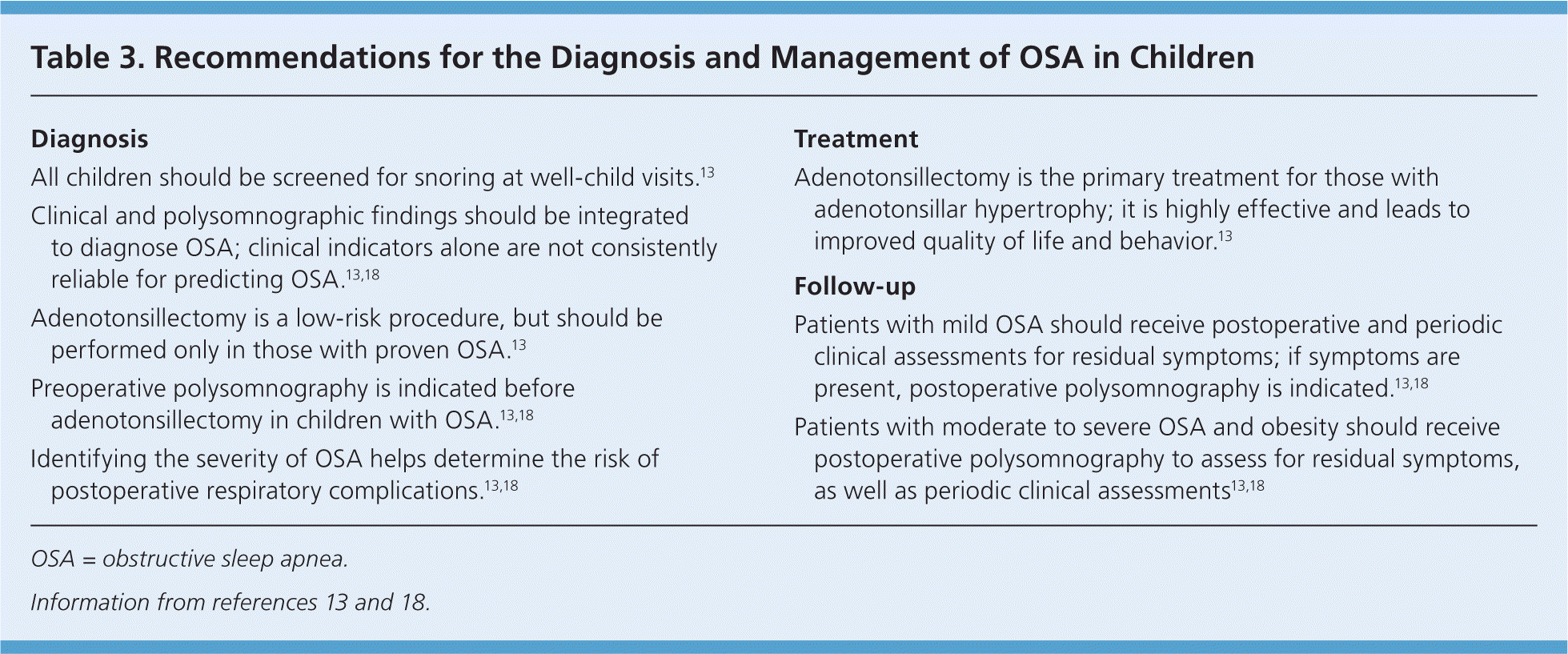
| Diagnosis |
| All children should be screened for snoring at well-child visits.13 |
| Clinical and polysomnographic findings should be integrated to diagnose OSA; clinical indicators alone are not consistently reliable for predicting OSA.13,18 |
| Adenotonsillectomy is a low-risk procedure, but should be performed only in those with proven OSA.13 |
| Preoperative polysomnography is indicated before adenotonsillectomy in children with OSA.13,18 |
| Identifying the severity of OSA helps determine the risk of postoperative respiratory complications.13,18 |
| Treatment |
| Adenotonsillectomy is the primary treatment for those with adenotonsillar hypertrophy; it is highly effective and leads to improved quality of life and behavior.13 |
| Follow-up |
| Patients with mild OSA should receive postoperative and periodic clinical assessments for residual symptoms; if symptoms are present, postoperative polysomnography is indicated.13,18 |
| Patients with moderate to severe OSA and obesity should receive postoperative polysomnography to assess for residual symptoms, as well as periodic clinical assessments13,18 |
Weight loss interventions have demonstrated benefits of reducing the severity of OSA and should be initiated in all children who are overweight or obese.13 Continuous positive airway pressure should be offered to those with residual OSA symptoms or if adenotonsillectomy was not performed. There is limited evidence to support the use of intranasal corticosteroids for children with mild OSA or with residual mild OSA following adenotonsil-lectomy.60 Although rapid maxillary expansion (i.e., use of an orthodontic device that widens the upper jaw) and montelukast (Singulair) are sometimes recommended, no clinical trial evidence supports the use of these treatments for OSA in children.
Parasomnias
Parasomnias such as sleepwalking (somnambulism), sleep talking (somniloquy), confusional arousals, sleep terrors, and nightmares affect up to 50% of children.12 They are defined as undesirable events that accompany sleep and typically occur during sleep-wake transitions.12 They are additionally characterized by complex, awake-like activity by the child that appears purposeful but lacks meaningful interaction with his or her environment. Associated features include confusion, automatic behaviors, difficulty awakening, amnesia, and rapid return to full sleep after the event.
Most parasomnias, such as sleepwalking, sleep talking, confusional arousals, and sleep terrors, occur in the first half of the sleep period during slow wave sleep; children typically have no memory of the event. In contrast, nightmares typically occur in the last half of the sleep period during rapid eye movement sleep, with children able to remember the event. It is important to note that the symptoms and timing of nocturnal seizures can overlap with parasomnias. Physicians should inquire about repetitive stereotypic behaviors and odd posturing that could represent nocturnal seizures.12
Genetically predisposed individuals are susceptible to precipitating factors, contributing to the development of parasomnias. Precipitating factors include insufficient sleep and disorders causing partial awakenings from sleep. OSA is a common trigger for parasomnias, and a review of studies showed that more than one-half of children referred for sleep terrors or sleepwalking also had OSA.21 Other triggers may include periodic limb movement disorder, gastroesophageal reflux disease, forced awakenings, and certain medications.12,21
Parasomnias often resolve spontaneously by adolescence; however, 4% of persons will have recurring events.12,22 Treatment centers on reassurance, reducing precipitating factors, and increasing total sleep times.21,23,61 When it is appropriate, parents should be counseled about safety measures (e.g., locking doors and windows, using motion alarms, clearing the floor of toys, placing the mattress on the floor). Children who exhibit atypical, harmful, or violent behaviors or who are unresponsive to conservative treatments should be referred for further evaluation.
Behavioral Insomnia of Childhood
Behavioral insomnia of childhood is characterized by a learned inability to fall and/or stay asleep; the estimated prevalence is 10% to 30%.12,24 The condition is divided into the sleep-onset association type and the limit-setting type. The sleep-onset association type is characterized by the child's inability or unwillingness to fall asleep or return to sleep in the absence of specific conditions, such as a parent rocking the child to sleep.12 The limit-setting type occurs when parents fail to set appropriate limits, such as when the parents allow the child to sleep in their bed when the child refuses to sleep.12 Most children with behavioral insomnia of childhood have features of both types.
Prevention is the best treatment for behavioral insomnia of childhood. Physicians should educate parents on normal sleep patterns, good sleep hygiene, realistic expectations, setting boundaries, and sleep plans. These plans should focus on regular and consistent feedings, nap times, bedtime routines, and sleep-wake times. Infants are more likely to become self-soothers (fall asleep on their own) when consistently placed in the crib awake vs. already asleep.62,63 Creating a regular routine will establish expectations, and the child will eventually learn how to fall asleep on his or her own. Extinction techniques (placing the child in bed and ignoring him or her until the morning, or for a set period) are effective in the treatment of this disorder.25–28 There are various extinction techniques, and no single method is superior. Techniques for managing behavioral insomnia of childhood are summarized in Table 4.27 Sleep or sedating medications are ineffective for the treatment of this disorder.29,30

| Treatment technique | Description | |
|---|---|---|
| Parental education | Parents are taught about good sleep practices, such as consistent feedings, nap times, bedtime routines, regular sleep-wake times, and placing the child in bed drowsy but awake. | |
| Unmodified extinction | The child is placed in bed at a predetermined bedtime. | |
| The child's crying, calls for the parents, and tantrums are ignored until the following morning, although significant cries for suspected injuries or illnesses are not ignored. | ||
| Cries are ignored to prevent reinforcing negative learned behavior (e.g., crying is rewarded with parental response/presence). | ||
| This technique can be difficult and distressing for parents. | ||
| Modified version for decreased parental distress: | ||
| A parent stays in the child's room, but follows the same technique. | ||
| Graduated extinction | This is fundamentally the same as unmodified extinction, but with scheduled “check-ins.” | |
| A parent checks on the child on a fixed schedule (e.g., every 10 minutes) or in gradually increased intervals (e.g., first check-in after five minutes, second check-in after 10 minutes). | ||
| Parental interactions with the child are calming and positive, but last no more than one minute at a time. | ||
| Positive bedtime routines/faded bedtime with response cost | Positive bedtime routines: Relaxing/calming activities are implemented before bedtime (e.g., bedtime stories). | |
| Faded bedtime: Bedtime is delayed until the predicted time of sleep onset to decrease the time the child spends in bed awake. | ||
| Response cost: The child is removed from bed for a specific amount of time if sleep onset does not occur within the desired period. | ||
| Scheduled awakenings | Parents must document the pattern of nighttime awakenings. | |
| The child is awakened before the normally predicted nighttime awakening, and the number of scheduled awakenings is slowly decreased over time. | ||
Delayed Sleep Phase Disorder
The master circadian clock, located within the suprachiasmatic nucleus, controls the timing of sleep and cycles approximately every 24 hours in most individuals.31 The discrepancy between the internal clock and the external world requires continuous “resetting” by time cues, such as light, melatonin, physical activity, body temperature, and meals. Light is the most powerful of these entrainers. Inappropriate timing of light exposure can alter the circadian rhythm. For example, light exposure before bedtime can suppress melatonin and ultimately delay sleep onset.31,32
In children with delayed sleep phase disorder, habitual sleep-wake times are delayed by at least two hours compared with socially acceptable times.12 The disorder is more common during adolescence when the circadian rhythm is thought to lengthen and the child becomes more social.31,64 The prevalence in adolescents is 7% to 16%.12,31,34 The disorder is diagnosed using patient history and documentation of sleep and wake times on a sleep diary or log. Parental concerns usually focus on late bedtimes (2 a.m. or later), sleeping in, difficulty awakening, and school tardiness. However, frequent nighttime awakenings are unusual, and sleep architecture is usually normal (Figure 2).
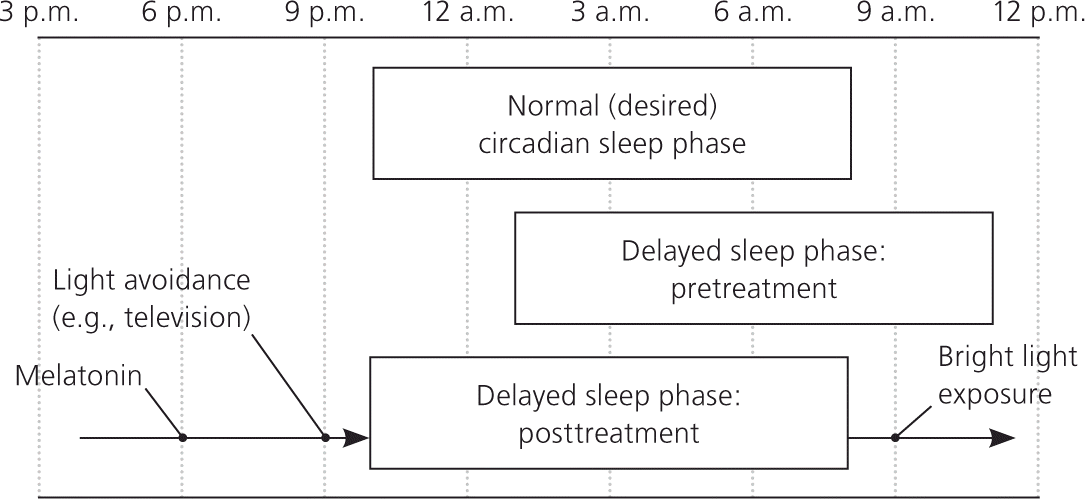
Treatment focuses on aligning the circadian rhythm with desired sleep-wake times. As in all sleep disorders, maintaining a regular sleep-wake cycle and practicing good sleep hygiene are the foundation of treatment. It is important to avoid bright lights before bedtime. Removing all light-emitting devices (e.g., electronics, portable media, tablet computers, cell phones) from the bedroom may be beneficial. Bright light therapy used for the first one to two hours after awakening may also be beneficial and will advance the circadian rhythm.31 There is strong evidence that melatonin supplementation (0.3 to 5 mg given 1.5 to 6.5 hours before desired bedtime) is an effective treatment for delayed sleep phase disorder, although the exact dose or timing has not been well established.36–38
Restless Legs Syndrome
The rate of restless legs syndrome in children is unclear, but limited studies suggest a prevalence of 2%.12,39 The condition is characterized by an unpleasant sensation in the legs, with the urge to move the legs starting in the evening.12 Rest worsens symptoms, and movement provides some relief. Other symptoms include difficulty falling asleep, bedtime resistance, “growing pains,” and symptoms similar to those of attention-deficit/hyperactivity disorder.12,40,42,43 The condition in children is associated with negative behavior and mood, and decreased cognition and attention, and it is more common in children with attention-deficit/hyperactivity disorder.12,39,42
Dopamine dysfunction, genetics, and iron deficiency are thought to play a role in the pathogenesis of restless legs syndrome.45–48 Additionally, symptoms may be exacerbated by excessive or inadequate physical activity or the use of caffeine, nicotine, antihistamines, selective serotonin reuptake inhibitors, or tricyclic antidepressants.47
Diagnosing restless legs syndrome in children can be challenging because they may be unable to describe the core symptoms. A diagnosis can be made if the history is consistent with the condition and at least two of the following are present: a sleep disturbance, a first-degree relative has the condition, or five or more periodic limb movements per hour of sleep during polysomnography.12 Once restless legs syndrome is diagnosed, conservative treatment includes avoiding exacerbating factors.47 Because iron deficiency is common in children, measuring the ferritin level is reasonable.49,50 Iron replacement should be initiated if ferritin levels are less than 50 mcg per L, and they should be rechecked in three months.46,48 There are no medications approved for treating restless legs syndrome in children. Patients with symptoms that do not respond to conservative treatments should be referred for further evaluation.
Data Sources: We searched PubMed using standard search and MESH search terms. The American Academy of Sleep Medicine, American Academy of Pediatrics, American Heart Association, and American Thoracic Society were searched for guidelines and recommendations. In addition, a set of references from Essential Evidence Plus were reviewed and cited as applicable. Search dates: March 2011 through November 2013.
