
Am Fam Physician. 2014;89(6):445-450
A more recent article on long-acting reversible contraception is available.
Related editorial: Optimal Use of IUDs: Why Aren't We There Yet?
Related letters: Lidocaine for Pain Control During IUD Placement and Optimal Time for IUD Insertion After an STI
Patient information: A handout on this topic is available at https://familydoctor.org/familydoctor/en/prevention-wellness/sex-birth-control/birth-control/intrauterine-device-iud.html.
Author disclosure: No relevant financial affiliations.
Three intrauterine devices (IUDs) are available in the United States: the copper T 380A and two levonorgestrel-releasing IUDs, one that releases 20 mcg of levonorgestrel per 24 hours, and one that releases 14 mcg per 24 hours. All are safe and effective methods of contraception that work predominantly by prefertilization mechanisms. The copper T 380A IUD may be placed in nonpregnant women at any time in the menstrual cycle. The prescribing information for the 20- and 14-mcg levonorgestrel-releasing IUDs advises that insertion occur during the first seven days of menses. Insertion immediately after vaginal or cesarean delivery may be considered with the copper T 380A and the 20-mcg levonorgestrel-releasing IUDs; however, expulsion rates are higher than with delayed postpartum insertion. The prescribing information for both levonorgestrel-releasing IUDs advises a waiting period of six weeks postpartum or following second-trimester pregnancy loss. Current guidelines indicate that IUDs are acceptable for use in nulliparous women, in adolescents, and in women who are breastfeeding. They may also be used in women who have a history of sexually transmitted infection, although screening is recommended. IUDs should not be inserted for at least three months after resolution of a sexually transmitted infection. Neither antibiotic prophylaxis nor misoprostol use before IUD insertion is beneficial. If pregnancy occurs, the IUD should be removed if feasible. Possible side effects of levonorgestrel-releasing IUDs include headaches, nausea, hair loss, breast tenderness, depression, decreased libido, ovarian cysts, oligomenorrhea, and amenorrhea. The main side effect of the copper T 380A IUD is increased menstrual bleeding, which may continue even with long-term use.
The intrauterine device (IUD) is a safe and highly effective means of contraception. Three intrauterine devices are available in the United States, the copper T 380A (Paragard) and two levonorgestrel-releasing IUDs (LNG-IUDs), one that releases 20 mcg of levonorgestrel per 24 hours (Mirena) and a new low-dose device that releases 14 mcg per 24 hours (Skyla).1–4 Failure rates within the first year after insertion are 0.6% to 0.8% for the copper T 380A IUD, 0.2% for the 20-mcg LNG-IUD, and 0.9% for the 14-mcg LNG-IUD.4,5 In contrast to that of many forms of contraception, the effectiveness of IUDs is not heavily dependent on user compliance.5
There is a normal return to fertility after discontinuation of the copper T 380A IUD or the 20-mcg LNG-IUD, with a pregnancy rate of 82% one year after device removal and 89% two years after device removal.6 Clinical trials for the 14-mcg LNG-IUD have found that 77% of women attempting to become pregnant within the first year after IUD removal are successful.4 Even with increasing costs of IUDs, they are one of the most cost-effective forms of long-term contraception.7,8 When discussing the mechanism of IUDs as part of the informed consent process, patients may be told that although prefertilization and postfertilization mechanisms may both contribute to the contraceptive effectiveness of IUDs, research suggests that the majority of effects occur prefertilization.1,9,10
This article focuses on updated considerations and guidelines for recommending and prescribing IUDs. A previous article in American Family Physician provides an overview of insertion and removal techniques.11
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Nulliparous women and adolescents can be offered an IUD, although the 20-mcg per 24 hours levonorgestrel-releasing IUD (Mirena) is not approved by the U.S. Food and Drug Administration for use in nulliparous women. | C | 1, 3, 4, 26 |
| Women who are at high risk of STIs but have no active signs or symptoms of genital tract STI should be tested for STIs at the time of IUD insertion. Insertion of the IUD may occur on the same day as STI testing, without waiting for test results. If results are subsequently found to be positive, treatment can be administered at that time and the IUD left in place. | C | 1, 22–25 |
| For women with a known STI that causes cervical infection, it is recommended that IUD insertion be delayed for at least three months after resolution of the infection. | C | 1 |
| Prophylactic antibiotics should not routinely be administered before IUD insertion. Antibiotic prophylaxis does not have a major effect on reducing the risk of pelvic infection, and does not alter the need for IUD removal in the months after insertion. | B | 1, 30, 31 |
| Misoprostol (Cytotec) should not be administered before IUD insertion. Although an earlier study showed easier insertion with misoprostol, subsequent studies showed no benefit and increased side effects. | B | 32–35 |
| If a woman with an IUD becomes pregnant, the IUD should be removed. | C | 1, 3, 4 |
Timing of Insertion
The copper T 380A IUD may be placed at any time during the menstrual cycle, as long as the patient is not pregnant.1,2 The prescribing information for the 14- and 20-mcg LNG-IUDs recommends insertion during the first seven days of the menstrual cycle.3,4 Placement of the copper T 380A IUD or the 20-mcg LNG-IUD is also considered safe and effective immediately after vaginal or cesarean delivery (within 10 minutes after placental separation), although the risk of expulsion is considerably higher than if insertion is delayed.1,12–15 One study comparing placement of the LNG-IUD immediately after placental delivery vs. six months postpartum found expulsion rates of 24% and 4%, respectively.13 Two other studies comparing expulsion rates with immediate and delayed insertion of the copper T 380A IUD following delivery (vaginal or cesarean) found expulsion rates of 12% and 17%, respectively.14,15 Immediate insertion after a pregnancy loss in the second trimester has a higher risk of expulsion compared with loss in the first trimester.1 The prescribing information for both types of the LNG-IUD advises a waiting period of six weeks postpartum or following second-trimester pregnancy loss.3,4
Although the expulsion rate is lower, one disadvantage of delayed insertion, for example at the postpartum visit, is that some women may not return for the follow-up.1,13 Therefore, women who might benefit most from insertion immediately after delivery would be those who are less likely to return for insertion. When postpartum contraception is discussed at prenatal visits, patients should be counseled about the pros and cons of immediate vs. delayed IUD placement.
Women who develop peripartum infections, such as chorioamnionitis, endometritis, or puerperal sepsis, should not undergo IUD placement for a least three months postpartum.1 IUDs may be placed immediately after completion of a spontaneous or induced abortion, except in cases of septic abortion, in which a waiting period of at least three months is advised.16
Special Populations
NULLIPAROUS WOMEN
Many physicians are reluctant to recommend an IUD to nulliparous women because of perceived concerns about safety,17 and because earlier studies suggested that younger, nulliparous women have a higher risk of device expulsion.18 Although good-quality data on expulsion rates in nulliparous women are lacking, one study of the 20-mcg LNG-IUD found that the expulsion rate in nulliparous adolescents was lower than the overall expulsion rate for all the adolescents in the study (4% vs. 8%, respectively).19 Another study comparing IUD use in nulliparous and parous women found similar rates of complications, patient discontinuation, and expulsion (expulsion rates were 0% to 0.2% per year for LNG-IUDs and 0% to 1.2% per year for copper IUDs).20 A third study found that although most nulliparous women reported pain during insertion of an LNG-IUD, they had continuation rates similar to those of women using oral contraceptives, confirming that the device is well tolerated in nulliparous women.21
The U.S. Medical Eligibility Criteria (USMEC) for Contraceptive Use guidelines, developed by the World Health Organization and adapted by the Centers for Disease Control and Prevention, assign the 20-mcg LNG-IUD and the copper T 380A IUD a category 2 rating for use in nulliparous women, which indicates that the advantages generally outweigh the theoretical or proven risks.1,22 The 14-mcg LNG-IUD was not available when the USMEC guidelines were written. Only the copper T 380A and the 14-mcg LNG-IUD are approved by the U.S. Food and Drug Administration for nulliparous women.1,3,4
Regardless of the IUD selected or the parity of the patient, clinicians should attempt safe, fundal placement to lower the risk of displacement and expulsion. Patients should be taught how to check for proper positioning of the IUD strings.11
WOMEN AT RISK OF STI
A history of sexually transmitted infection (STI) does not preclude IUD insertion.1,23 In a study of patients in an urban university clinic, IUDs were used safely in women with a history of STI, and the incidence of STI in these women decreased following IUD insertion.23 However, clinicians should screen patients following Centers for Disease Control and Prevention guidelines for all women.24 For women with a known STI, it is recommended that IUD insertion be delayed for at least three months after resolution of the infection. Women should also be rescreened for STI three to six months after treatment.1
If a woman does not have a known STI or active signs or symptoms of a genital tract STI, it is safe to screen for STI on the same day as IUD placement, although the rate of pelvic inflammatory disease will be slightly increased if it turns out that an STI was present at the time of IUD insertion (0% to 5% with STI present vs. 0% to 2% without).1,25 The prescribing information for the LNG-IUDs states that it is usually appropriate to remove an IUD if an STI is diagnosed; however, the USMEC guidelines state that in a patient who tests positive for an STI but has no symptoms, antibiotics may be prescribed and, if clinically appropriate, the IUD may be left in place.1,3,4,22
ADOLESCENTS
The use of IUDs in adolescents (i.e., females from menarche to younger than 21 years) has been questioned because of the increased prevalence of STIs in this group, and the resulting increased risk of pelvic inflammatory disease and subsequent infertility. A 2012 American College of Obstetricians and Gynecologists committee opinion states that IUDs do not increase the risk of pelvic inflammatory disease or decrease future fertility in adolescents.26 Furthermore, a recent study on the effectiveness of long-acting contraception found the IUD to be more effective than the contraceptive pill, patch, or ring for prevention of pregnancy, including in adolescents.27 The USMEC guidelines state that the advantages of using the IUD in adolescents generally outweigh the risks.22
MEDICAL CONDITIONS
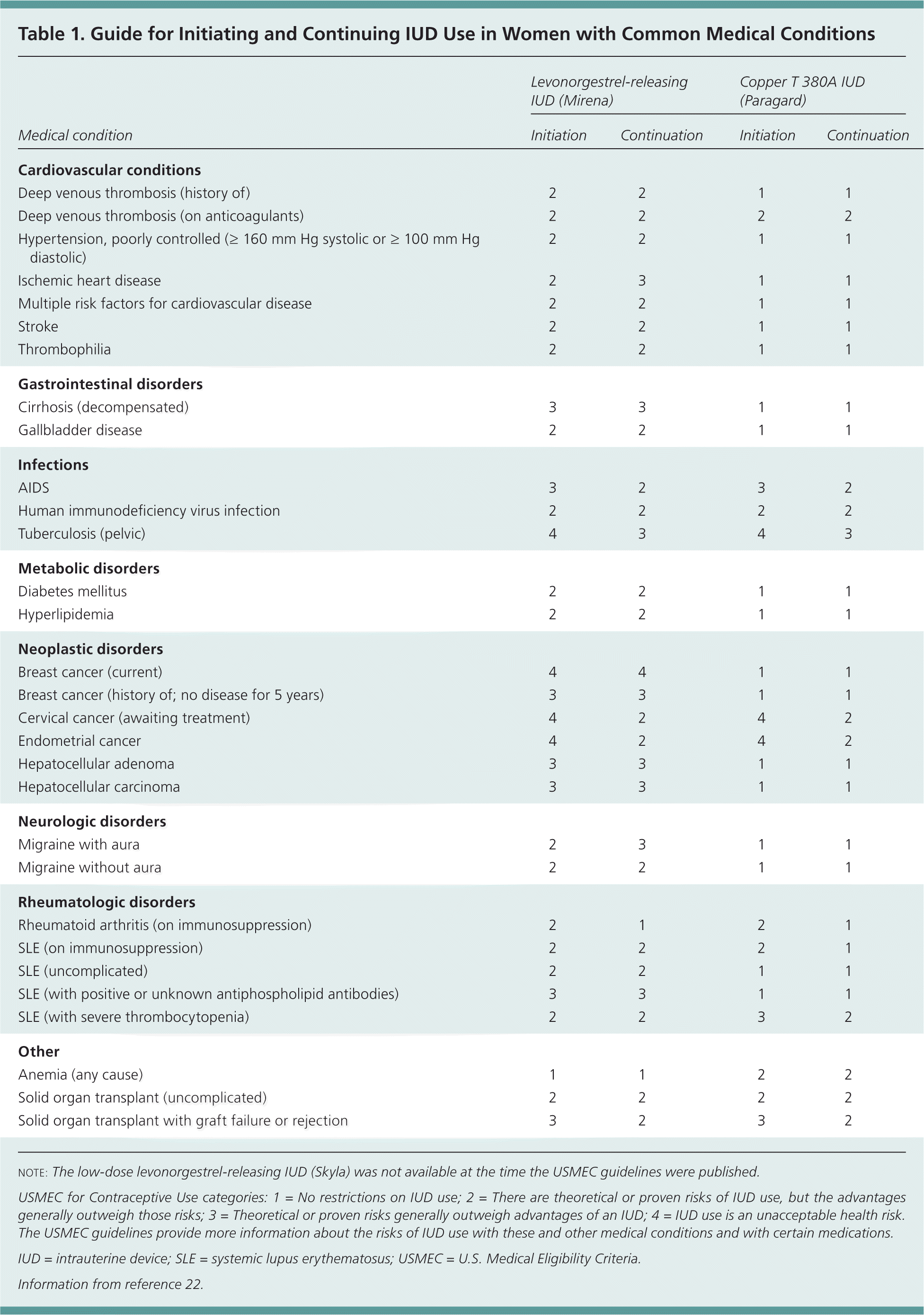
| Medical condition | Levonorgestrel-releasing IUD (Mirena) | Copper T 380A IUD (Paragard) | ||
|---|---|---|---|---|
| Initiation | Continuation | Initiation | Continuation | |
| Cardiovascular conditions | ||||
| Deep venous thrombosis (history of) | 2 | 2 | 1 | 1 |
| Deep venous thrombosis (on anticoagulants) | 2 | 2 | 2 | 2 |
| Hypertension, poorly controlled (≥ 160 mm Hg systolic or ≥ 100 mm Hg diastolic) | 2 | 2 | 1 | 1 |
| Ischemic heart disease | 2 | 3 | 1 | 1 |
| Multiple risk factors for cardiovascular disease | 2 | 2 | 1 | 1 |
| Stroke | 2 | 2 | 1 | 1 |
| Thrombophilia | 2 | 2 | 1 | 1 |
| Gastrointestinal disorders | ||||
| Cirrhosis (decompensated) | 3 | 3 | 1 | 1 |
| Gallbladder disease | 2 | 2 | 1 | 1 |
| Infections | ||||
| AIDS | 3 | 2 | 3 | 2 |
| Human immunodeficiency virus infection | 2 | 2 | 2 | 2 |
| Tuberculosis (pelvic) | 4 | 3 | 4 | 3 |
| Metabolic disorders | ||||
| Diabetes mellitus | 2 | 2 | 1 | 1 |
| Hyperlipidemia | 2 | 2 | 1 | 1 |
| Neoplastic disorders | ||||
| Breast cancer (current) | 4 | 4 | 1 | 1 |
| Breast cancer (history of; no disease for 5 years) | 3 | 3 | 1 | 1 |
| Cervical cancer (awaiting treatment) | 4 | 2 | 4 | 2 |
| Endometrial cancer | 4 | 2 | 4 | 2 |
| Hepatocellular adenoma | 3 | 3 | 1 | 1 |
| Hepatocellular carcinoma | 3 | 3 | 1 | 1 |
| Neurologic disorders | ||||
| Migraine with aura | 2 | 3 | 1 | 1 |
| Migraine without aura | 2 | 2 | 1 | 1 |
| Rheumatologic disorders | ||||
| Rheumatoid arthritis (on immunosuppression) | 2 | 1 | 2 | 1 |
| SLE (on immunosuppression) | 2 | 2 | 2 | 1 |
| SLE (uncomplicated) | 2 | 2 | 1 | 1 |
| SLE (with positive or unknown antiphospholipid antibodies) | 3 | 3 | 1 | 1 |
| SLE (with severe thrombocytopenia) | 2 | 2 | 3 | 2 |
| Other | ||||
| Anemia (any cause) | 1 | 1 | 2 | 2 |
| Solid organ transplant (uncomplicated) | 2 | 2 | 2 | 2 |
| Solid organ transplant with graft failure or rejection | 3 | 2 | 3 | 2 |
Other Considerations
BREASTFEEDING
Use of IUDs is considered acceptable in women who are breastfeeding, although there are limited data on whether rates of breastfeeding success differ among women using the copper T 380A vs. an LNG-IUD. One study found no difference in infant growth and development or in overall breastfeeding success between the two types of IUD.1,28 The American Academy of Family Physicians supports the use of IUDs in women who are breastfeeding.29
ANTIBIOTIC PROPHYLAXIS BEFORE IUD INSERTION
There is a slightly increased risk of pelvic inflammatory disease within the first 20 days after insertion, likely related to insertion technique, although the risk is low thereafter.1,30 Antibiotic prophylaxis before or at the time of IUD insertion does not have a major effect on reducing the risk of pelvic infection, and does not alter the need for IUD removal in the months after insertion,30 even in women at risk of STIs.1,30,31
USE OF MISOPROSTOL BEFORE INSERTION
A 2007 study suggested that the use of misoprostol (Cytotec) before IUD insertion allowed for easier insertion.32 However, more recent studies show no benefit and increased side effects with misoprostol.33–35 The American College of Obstetricians and Gynecologists makes no recommendation regarding the use of misoprostol before IUD insertion.
PREGNANCY IN WOMEN WITH AN IUD IN PLACE
The risk of ectopic pregnancy is higher in women who become pregnant with an IUD in place, compared with women who do not have an IUD.1 However, because IUDs are highly effective at preventing pregnancy, ectopic pregnancies occur less often in IUD users than in women using other methods of contraception or no contraception.1,36
Once pregnancy is confirmed in a woman with an IUD, ectopic pregnancy should be excluded and the IUD removed.1,3,4 If the strings are not visible, ultrasonography can be used to confirm whether the IUD is present in the uterus. Invasive procedures, such as hysteroscopy, are not recommended for IUD removal during pregnancy.1 Patients who become pregnant with an IUD in place should be informed that there is a substantial risk of pregnancy loss even when the IUD is removed. One study found that among 89 women who became pregnant with an IUD in place and desired pregnancy continuation, 40% had spontaneous abortion following IUD removal.37 IUD removal is recommended because pregnancy loss and complications are less likely if the IUD is removed than if it is left in place. Later pregnancy complications, including placental abruption, placenta previa, preterm delivery, low birth weight, chorioamnionitis, and need for cesarean delivery, are also more likely in women who become pregnant with an IUD in place.1
SIDE EFFECTS
All types of IUDs are well tolerated, with continuation rates higher than those for all other forms of reversible contraception except the contraceptive implant (78% for the copper T 380A, 80% for the 20-mcg LNG-IUD, and 82% for the 14-mcg LNG-IUD).3–5 However, there are possible side effects that patients should be aware of.
Side effects of the LNG-IUD are similar to those of other progestin-based contraceptives and include headaches, nausea, hair loss, breast tenderness, depression, decreased libido, and ovarian cysts.3,4,38 Women who use the 14-mcg or 20-mcg LNG-IUD also experience vulvovaginitis at rates of 20.2% and less than 5%, respectively, as well as abdominal/pelvic pain at rates of 18.9% and 12.8%, respectively.3,4 Women may have amenorrhea or irregular spotting throughout use of the LNG-IUD, although the amount of bleeding decreases for most women the longer the IUD is in place because of thinning of the endometrium.1 Up to 70% of women using the 20-mcg LNG-IUD report oligomenorrhea or amenorrhea after two years of use.1,3,39,40 Because the 20-mcg LNG-IUD reduces endometrial thickness, it has been successfully used for the treatment of menorrhagia.41–44
The copper T 380A IUD can cause irregular, heavy bleeding. Unlike the LNG-IUD, bleeding, including painful intermenstrual bleeding, may continue throughout use.1,2,45 Nonetheless, discontinuation rates are similar between the IUD types.4,46 Because the copper device does not contain hormonal agents, it does not cause the progestin-related side effects possible with LNG-IUDs.1
Data Sources: A PubMed search was completed using the terms IUD and intrauterine device in combination with nulliparous, sexually transmitted infection, adolescent, levonorgestrel, copper T 380A, post-partum insertion, misoprostol, and side effects. The search included practice guidelines, reviews, and randomized controlled trials. Guidelines reviewed included those from the Agency for Healthcare Research and Quality's National Guideline Clearinghouse, Centers for Disease Control and Prevention, the World Health Organization's U.S. Medical Eligibility Criteria for Contraceptive Use, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists. Package inserts for all IUDs available in the United States were also reviewed. Search dates: August 2011 to November 2013.
EDITOR'S NOTE: Barry Weiss, MD, is medical editor of FP Essentials and is an associate medical editor for American Family Physician.
