
Am Fam Physician. 2014;89(7):545-550
Related letter: Reduced Effectiveness of Emergency Contraception in Women with Increased BMI
Patient information: See related handout on emergency contraception written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Emergency contraception decreases the risk of unintended pregnancy after unprotected sexual intercourse or after suspected failure of routine contraception (e.g., a condom breaking). Oral methods include combined contraceptive pills (i.e., Yuzpe method), single- or split-dose levonorgestrel, and ulipristal. The Yuzpe method and levonorgestrel are U.S. Food and Drug Administration–approved for use 72 hours postcoitus, whereas the newest method, ulipristal, is approved for up to 120 hours postcoitus. The copper intrauterine device may be used as emergency contraception up to seven days after unprotected intercourse. It is nonhormonal and has the added benefit of long-term contraception. Advanced provision of emergency contraception may be useful for all patients, and for persons using ulipristal because it is available only by prescription. Physicians should counsel patients on the use and effectiveness of emergency contraception, the methods available, and the benefits of routine and consistent contraception use.
Emergency contraception has the potential to prevent more than 3 million unintended pregnancies in the United States each year.1,2 Each method has a different level of effectiveness depending on the timeliness of use. Although it can be effective up to 120 hours postcoitus, it is most effective within the first 12 hours after unprotected intercourse or if the routine contraception method fails (e.g., a condom breaking).2,3
The use of emergency contraception has increased significantly, with more than 5 million women reporting use at least once between 2006 and 2008.1 This may be largely because of increased availability. In 2006, levonorgestrel was approved for over-the-counter use in women 18 years and older, and in 2009, the age was lowered to include those 17 years of age.4,5 Most recently, in 2013, the U.S. Food and Drug Administration (FDA) announced that Plan B One-Step, a single-dose levonorgestrel product, is available over the counter without age restrictions.6 However, the generic single-dose and the split-dose levonorgestrel are still behind the counter for those 17 years or older, and available by prescription only to those 16 years or younger.7
There are four methods of emergency contraception currently approved by the FDA: combined oral contraceptive pills (i.e., Yuzpe method), progestin-only pills containing levonorgestrel, ulipristal (Ella), and the copper intrauterine device (IUD; Paragard). Table 1 provides information on dosing and cost.8
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Ulipristal (Ella) is marginally more effective than levonorgestrel at preventing unintended pregnancy within 72 hours postcoitus. Levonorgestrel appears to be equally effective in the single- and split-dose regimens. | A | 12 |
| The copper intrauterine device is the most effective method of emergency contraception and can be considered by women who are not at high risk of sexually transmitted infections and who desire long-term contraception. | A | 12 |
| There is no absolute contraindication to the use of oral emergency contraception, with the exception of pregnancy. | C | 28 |
| Advanced provision of emergency contraception increases the rate and timeliness of use, and does not increase the rate of sexually transmitted infections or change the use of routine contraceptive methods. | C | 33–35 |
| Recommendation | Sponsoring organization |
|---|---|
| Do not require a pelvic exam or other physical exam to prescribe oral contraceptive medications. | American Academy of Family Physicians |
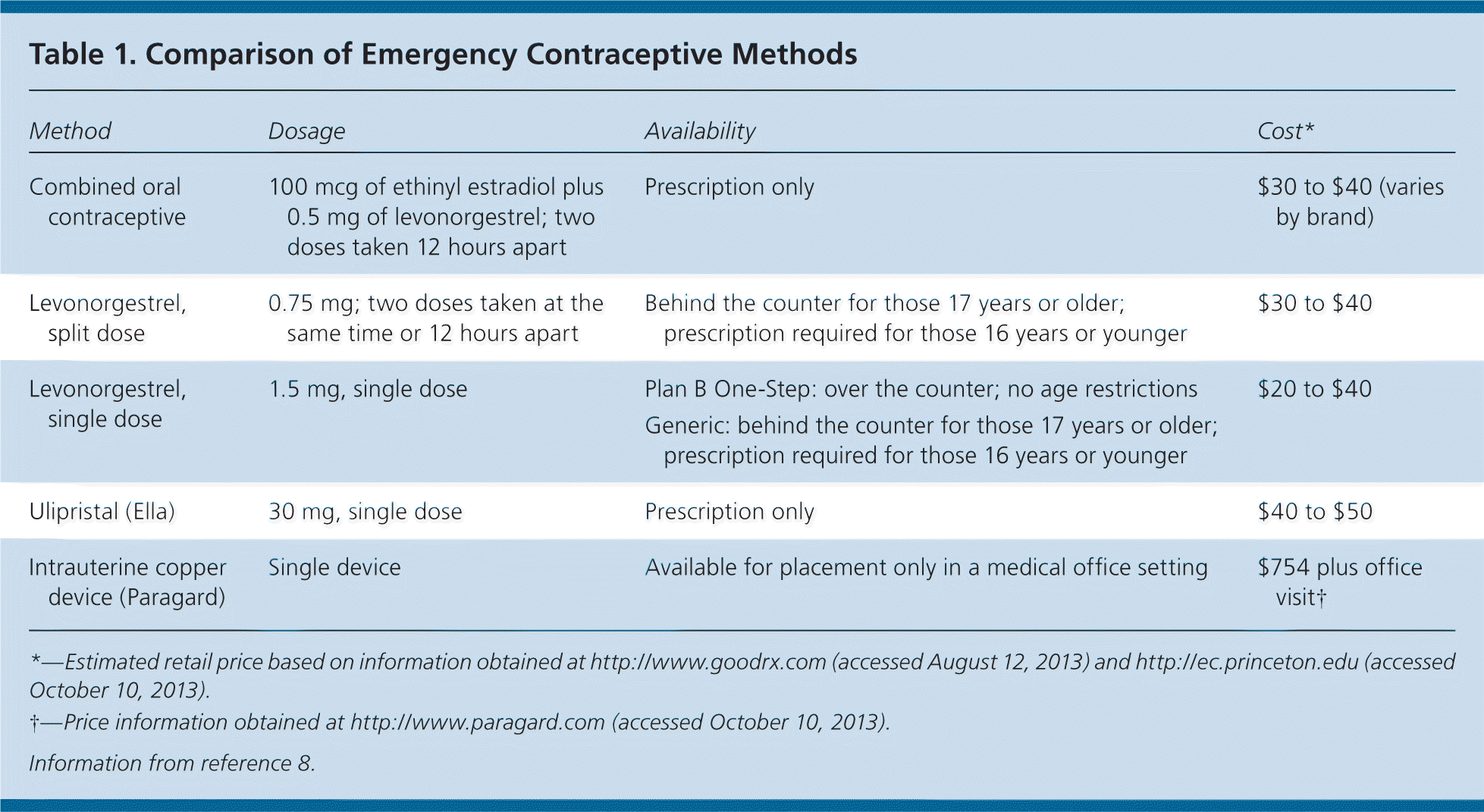
| Method | Dosage | Availability | Cost* |
|---|---|---|---|
| Combined oral contraceptive | 100 mcg of ethinyl estradiol plus 0.5 mg of levonorgestrel; two doses taken 12 hours apart | Prescription only | $30 to $40 (varies by brand) |
| Levonorgestrel, split dose | 0.75 mg; two doses taken at the same time or 12 hours apart | Behind the counter for those 17 years or older; prescription required for those 16 years or younger | $30 to $40 |
| Levonorgestrel, single dose | 1.5 mg, single dose | Plan B One-Step: over the counter; no age restrictions | $20 to $40 |
| Generic: behind the counter for those 17 years or older; prescription required for those 16 years or younger | |||
| Ulipristal (Ella) | 30 mg, single dose | Prescription only | $40 to $50 |
| Intrauterine copper device (Paragard) | Single device | Available for placement only in a medical office setting | $754 plus office visit† |
Yuzpe Method
The Yuzpe method consists of two doses of a combination estrogen/progestin oral contraceptive (100 mcg ethinyl estradiol and 1 mg dl-norgestrel [equivalent to 0.5 mg levonorgestrel]) taken 12 hours apart.9 This regimen offers a convenient method for patients to use pills they already have. Dosing regimens for several common combination oral contraceptives are listed in Table 2.8 This method is 56% to 86% effective, depending on the timeliness of use after unprotected intercourse; it is most effective when used within 72 hours, and is less effective when used 72 to 120 hours after unprotected intercourse.3
Because women present for emergency contraception at various times in their menstrual cycle, the expected pregnancy rate is 4% without the Yuzpe method and 2% with it.10 The number needed to treat to prevent one pregnancy is 50.10 This method works primarily by preventing ovulation, although it theoretically could prevent implantation.11 Nausea and vomiting are the most common adverse effects, and physicians may consider recommending an antiemetic before use.12,13
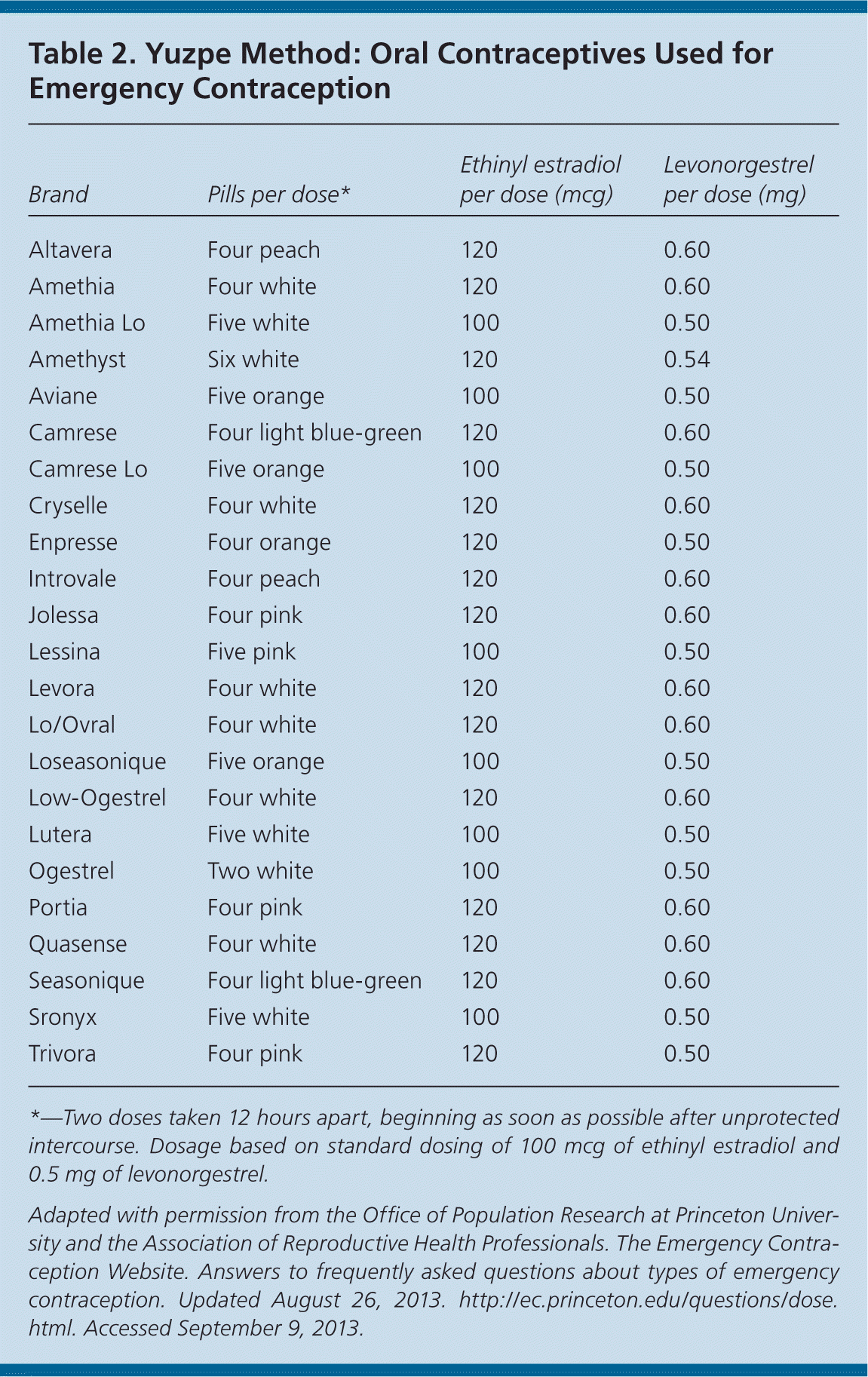
| Brand | Pills per dose* | Ethinyl estradiol per dose (mcg) | Levonorgestrel per dose (mg) |
|---|---|---|---|
| Altavera | Four peach | 120 | 0.60 |
| Amethia | Four white | 120 | 0.60 |
| Amethia Lo | Five white | 100 | 0.50 |
| Amethyst | Six white | 120 | 0.54 |
| Aviane | Five orange | 100 | 0.50 |
| Camrese | Four light blue-green | 120 | 0.60 |
| Camrese Lo | Five orange | 100 | 0.50 |
| Cryselle | Four white | 120 | 0.60 |
| Enpresse | Four orange | 120 | 0.50 |
| Introvale | Four peach | 120 | 0.60 |
| Jolessa | Four pink | 120 | 0.60 |
| Lessina | Five pink | 100 | 0.50 |
| Levora | Four white | 120 | 0.60 |
| Lo/Ovral | Four white | 120 | 0.60 |
| Loseasonique | Five orange | 100 | 0.50 |
| Low-Ogestrel | Four white | 120 | 0.60 |
| Lutera | Five white | 100 | 0.50 |
| Ogestrel | Two white | 100 | 0.50 |
| Portia | Four pink | 120 | 0.60 |
| Quasense | Four white | 120 | 0.60 |
| Seasonique | Four light blue-green | 120 | 0.60 |
| Sronyx | Five white | 100 | 0.50 |
| Trivora | Four pink | 120 | 0.50 |
Levonorgestrel
Levonorgestrel can be taken as a single dose of 1.5 mg or two 0.75-mg doses taken at the same time or 12 hours apart within 72 hours of unprotected intercourse.14,15 There is decreased effectiveness as time passes after unprotected intercourse. The effectiveness ranges from 58% to 79%.15,16 The expected pregnancy rate of 4% decreases to less than 2% after use of levonorgestrel.10 The number needed to treat to prevent one pregnancy is 43.10 Single-dose levonorgestrel has similar effectiveness as the split-dose regimen.12,17 Single and split dosing were found to be equally effective at preventing unintended pregnancy, even with additional acts of unprotected intercourse.12 Levonorgestrel works by interfering with the luteinizing hormone peak during the cycle.18,19 When levonorgestrel was given before the peak, it was effective at preventing ovulation, but it did not prevent pregnancy if ovulation and fertilization had already occurred.18,20 Levonorgestrel use may cause an earlier onset of menstrual bleeding, headache, fatigue, dizziness, back pain, and dysmenorrhea.15,21
Ulipristal
Available since December 2010, ulipristal is a progesterone receptor modulator and the newest FDA-approved medication for emergency contraception. The dosing is a single 30-mg tablet taken within 120 hours of unprotected intercourse.22 It is the first oral emergency contraception that has proven effective for late intake (i.e., 96 to 120 hours postcoitus).22 One study evaluated ulipristal at three postcoital time intervals of 48 to 72 hours, 72 to 96 hours, and 96 to 120 hours; effectiveness rates were 62%, 58%, and 75%, respectively.22 When taken within 72 hours, ulipristal has similar effectiveness to the single- and split-dose levonorgestrel; however, it maintains a higher degree of effectiveness between 72 and 120 hours.12,21 Ulipristal works by binding to progesterone receptors, and subsequently inhibiting or delaying ovulation. Unlike levonorgestrel, ulipristal is effective regardless of the luteinizing hormone peak.22 When taken before ovulation, it was more effective than levonorgestrel at preventing ovulation, and it delayed ovulation by as much as five days.23 Ulipristal can delay the onset of menses up to five days, and can also cause headache, fatigue, dizziness, back pain, and dysmenorrhea.21
Copper IUD
For patients desiring long-term contraception, as well as emergency contraception, a copper IUD may be placed up to seven days after unprotected intercourse.24,25 When placed after unprotected intercourse, the copper IUD has a failure rate of 0.09%.12,24 The copper IUD is nonhormonal and continuously releases copper into the uterine cavity.24,25 It prevents pregnancy by interfering with fertilization and preventing implantation. There is a lack of studies comparing its effectiveness with any of the aforementioned regimens.12 However, there are no increased risks associated with the copper IUD when used for emergency contraception compared with usual use.25 Table 3 compares the effectiveness of common methods of contraception with emergency contraception.3,12,16,26,27
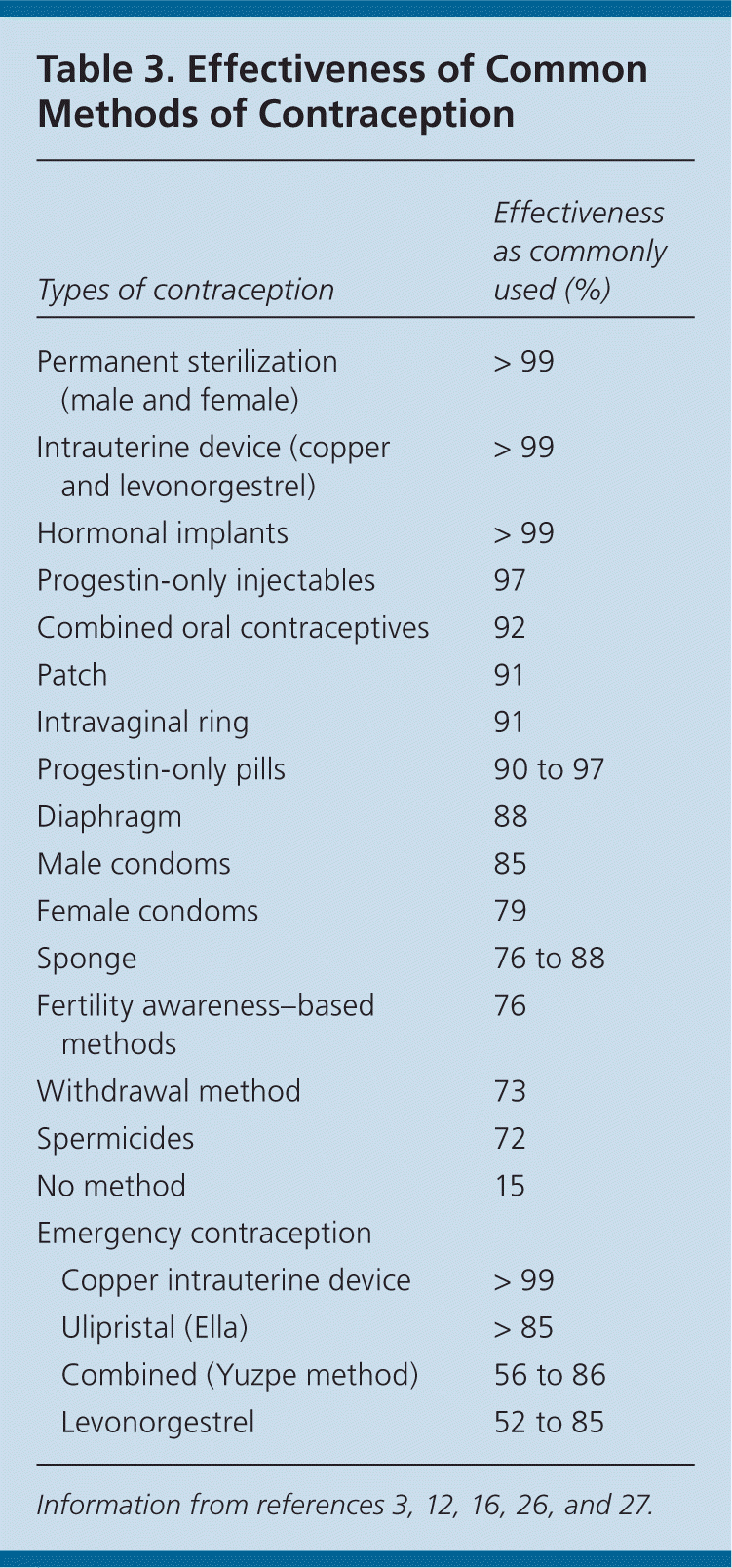
| Types of contraception | Effectiveness as commonly used (%) | |
|---|---|---|
| Permanent sterilization (male and female) | > 99 | |
| Intrauterine device (copper and levonorgestrel) | > 99 | |
| Hormonal implants | > 99 | |
| Progestin-only injectables | 97 | |
| Combined oral contraceptives | 92 | |
| Patch | 91 | |
| Intravaginal ring | 91 | |
| Progestin-only pills | 90 to 97 | |
| Diaphragm | 88 | |
| Male condoms | 85 | |
| Female condoms | 79 | |
| Sponge | 76 to 88 | |
| Fertility awareness–based methods | 76 | |
| Withdrawal method | 73 | |
| Spermicides | 72 | |
| No method | 15 | |
| Emergency contraception | ||
| Copper intrauterine device | > 99 | |
| Ulipristal (Ella) | > 85 | |
| Combined (Yuzpe method) | 56 to 86 | |
| Levonorgestrel | 52 to 85 | |
Teratogenicity and Contraindications
There have been no reported cases of birth defects from the use of oral emergency contraception. Investigation into the harms of high-dose contraception showed no risk to an already established pregnancy.12 According to the Centers for Disease Control and Prevention's U.S. Medical Eligibility Criteria for Contraceptive Use, 2010, no conditions strictly prohibit the use of oral emergency contraception, with the exception of pregnancy.28 Because the duration of use for emergency contraception is substantially less than for routine contraception, the potential risk of adverse effects is also reduced.28
The same contraindications exist for the copper IUD whether used as emergency or routine contraception.25 In cases of sexual assault, if the patient is considered at high risk of pelvic inflammatory disease, a copper IUD is not recommended.28 However, an IUD could be considered if the patient is adequately screened for sexually transmitted infections and if the insertion criteria are met as outlined in the Physician's Desk Reference.25
There is insufficient evidence to recommend against the use of emergency contraception in women with a body mass index greater than 30 kg per m2; however, preliminary studies show that women with obesity may have a significantly higher failure rate when using levonorgestrel or ulipristal.29
Resuming Contraception
Although emergency contraception reduces the risk of pregnancy following intercourse, routine contraception is most effective (Table 3).3,12,16,26,27 All patients who use levonorgestrel and ulipristal for emergency contraception should be encouraged to begin or continue a regular birth control method because a rapid return to fertility is expected.30,31 Patients may start using routine hormonal contraception according to usual prescribing methods immediately after the use of emergency contraception. The prescribing information for ulipristal recommends the use of a reliable barrier method of contraception with subsequent acts of intercourse in the same menstrual cycle because ulipristal may reduce the contraceptive action of hormonal contraception.30 Women who use any form of emergency contraception and do not start menses within seven days of the expected time should see a physician to be evaluated for possible pregnancy.30
Access
Access to emergency contraception was broadened in August 2006, when the FDA approved nonprescription access to levonorgestrel for women 18 years and older.4 In March 2009, a federal court issued an order directing the FDA to make levonorgestrel available to those 17 years or older without a prescription.5 Currently, there is a distinction between single- and split-dose levonorgestrel. Single-dose Plan B One-Step is now available over the counter without age restriction. The split-dose and generic single-dose options are still available behind the counter for those 17 years or older, and by prescription only for those 16 years or younger.7 Ulipristal is available by prescription only; the copper IUD is available for placement only in a medical office setting.
Eighteen states and the District of Columbia require hospitals or health care facilities to provide information about or to initiate emergency contraception therapy to women who have been sexually assaulted.32 These include Arkansas, California, Colorado, Connecticut, Hawaii, Illinois, Massachusetts, Minnesota, New Jersey, New Mexico, New York, Oregon, Pennsylvania, South Carolina, Texas, Utah, Washington, and Wisconsin.
Advanced Provision
Advanced provision of emergency contraception (i.e., providing patients with prescriptions or pills before the need for use) has been shown to increase the rate and timeliness of use in young women and adolescents.33–35 Advanced provision and use does not increase the rates of sexually transmitted infections or of unprotected intercourse, or change the use of routine contraception. It is useful for patients who prefer ulipristal and for adolescents younger than 17 years who use the single- or split-dose levonorgestrel. Despite allowing women and adolescents to use emergency contraception in a timelier manner, advanced provision has not led to decreased rates of pregnancy.33–35
Although some methods of emergency contraception are now available without a prescription, most patients do not know how to use it correctly or how to obtain it.36 The American Academy of Family Physicians' policy on contraceptive advice states that physicians should provide patient education and counseling to men and women to decrease the number of unwanted pregnancies. This includes information about abstinence, and the provision of routine and emergency contraception. It also includes the discussion of all forms of contraception, where to obtain them, and the reliability of each.37
Data Sources: A PubMed search was completed using key terms contraception, postcoital, and emergency. Also searched were the Cochrane database and National Guideline Clearinghouse database. Search dates: July and September 2011, and October 2013.
