
A more recent article on neonatal hyperbilirubinemia is available.
Am Fam Physician. 2014;89(11):873-878
Related editorial: Bilirubin Screening in Newborns: What Should We Do?
Author disclosure: No relevant financial affiliations.
Although neonatal jaundice is common, acute bilirubin encephalopathy and kernicterus (i.e., chronic bilirubin encephalopathy) are rare. Universal screening for neonatal hyperbilirubinemia is controversial. The American Academy of Pediatrics recommends universal screening with bilirubin levels or targeted screening based on risk factors. However, the U.S. Preventive Services Task Force and the American Academy of Family Physicians found insufficient evidence that screening improves outcomes. Universal screening may also increase rates of phototherapy, sometimes inappropriately. Younger gestational age and exclusive breastfeeding are the strongest risk factors for the development of hyperbilirubinemia. Infants who appear jaundiced should be evaluated by a risk score or by measurement of total serum or transcutaneous bilirubin. Phototherapy is an effective treatment for hyperbilirubinemia, but the number needed to treat varies widely depending on sex, gestational age, and time since delivery. If indicated, phototherapy should be initiated based on gestational age and risk factors. Exchange transfusion leads to complications in about 5% of treated infants and has a mortality rate of three or four per 1,000 infants. Infants who breastfeed exclusively—particularly those who consume inadequate calories—are at increased risk of hyperbilirubinemia. However, interrupting breastfeeding for the treatment of jaundice increases the risk of early discontinuation of breastfeeding. Encouragement from health care professionals is important to promote breastfeeding in these situations.
Neonatal jaundice affects up to 84% of term newborns1 and is the most common cause of hospital readmission in the neonatal period.2 Severe hyperbilirubinemia (total serum bilirubin [TSB] level of more than 20 mg per dL [342.1 μmol per L]) occurs in less than 2% of term infants and can lead to kernicterus (i.e., chronic bilirubin encephalopathy) and permanent neurodevelopmental delay.2 Therefore, it is important to systematically evaluate all infants for hyperbilirubinemia.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Phototherapy decreases the incidence of severe hyperbilirubinemia in newborns. | C | 5 |
| Phototherapy decreases the need for exchange transfusion in newborns with severe hyperbilirubinemia. | B | 3, 26 |
| Interrupting breastfeeding in an infant with jaundice decreases the chances of successful breastfeeding. | B | 28 |
Acute bilirubin encephalopathy develops in one in 10,000 infants and presents with hypertonia, arching, retrocollis, opisthotonos, fever, and high-pitched cry.2 Data on progression of acute bilirubin encephalopathy to kernicterus are limited, but one study found that 95% of infants with acute bilirubin encephalopathy had full resolution of symptoms, and 5% had evidence of kernicterus by the time of discharge.3 Kernicterus develops in one in 100,000 infants and manifests as athetoid cerebral palsy, auditory dysfunction, dental dysplasia, paralysis of upward gaze, and variable intellectual disability.
Risk factors for the development of severe hyperbilirubinemia include cephalhematoma or significant bruising, early gestational age, exclusive breastfeeding (especially unsuccessful breastfeeding and/or weight loss of 8% to 10%), isoimmune or other hemolytic anemia, and a sibling with a history of neonatal jaundice.4 In addition to hyperbilirubinemia, earlier gestational age, hemolysis, sepsis, and low birth weight are associated with the development of bilirubin encephalopathy. One study found that less than 5% of healthy term infants with a TSB level greater than 30 mg per dL (513.1 μmol per L) developed acute bilirubin encephalopathy or kernicterus.3
What Are the Current Recommendations on Screening for Hyperbilirubinemia?
The American Academy of Pediatrics recommends universal screening with TSB or transcutaneous bilirubin (TcB) levels, or targeted screening based on risk factors.5 Universal TSB/TcB screening can accurately identify infants whose TSB level is likely to exceed the 95th percentile for age.6,7 Some studies have found that the use of risk scores is as accurate as universal screening for predicting hyperbilirubinemia.8,9 A combination of universal screening and risk factor scoring seems to be the most effective method for identifying infants at risk of hyperbilirubinemia.1,7 A sample risk score is listed in Table 1.9
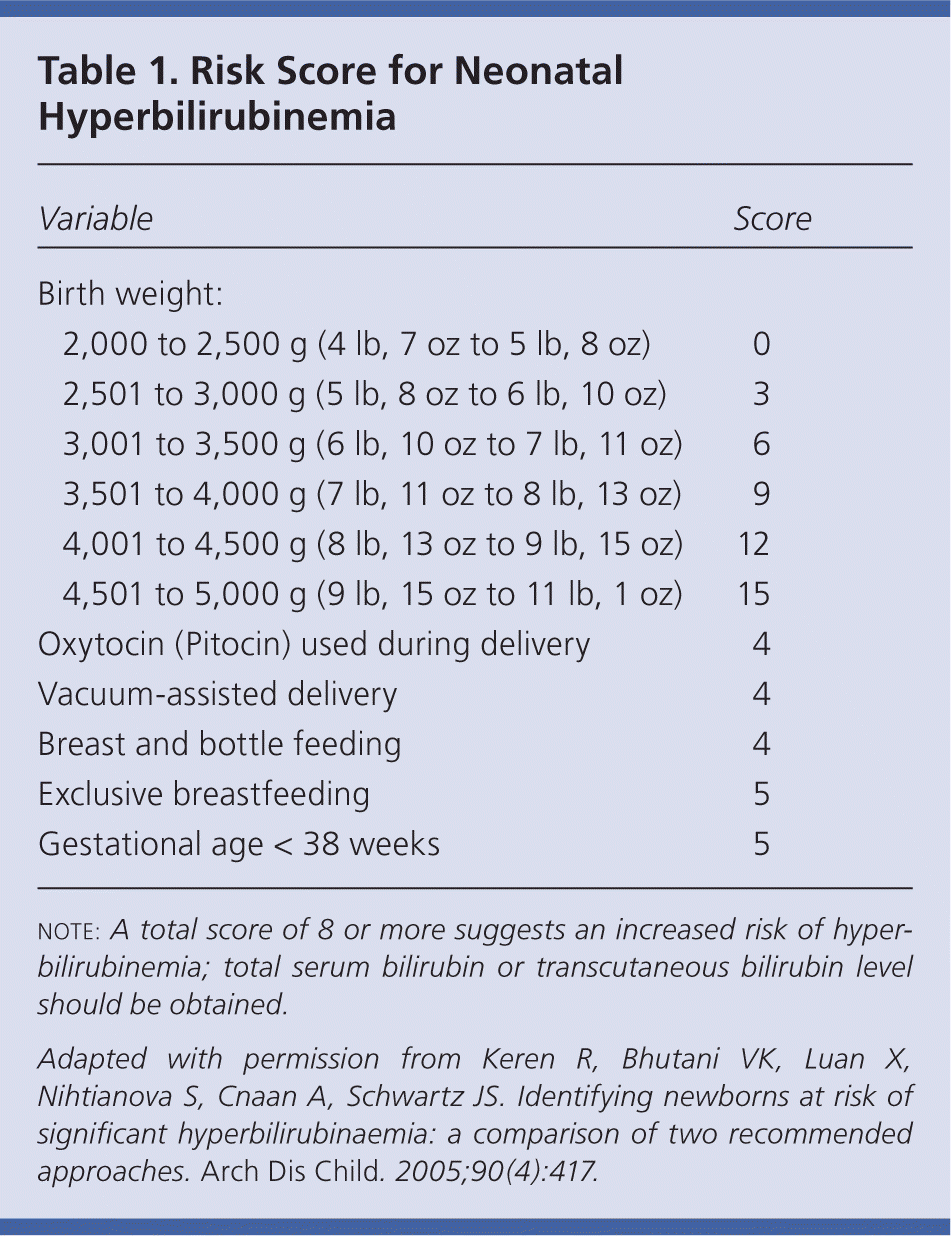
| Variable | Score | |
|---|---|---|
| Birth weight: | ||
| 2,000 to 2,500 g (4 lb, 7 oz to 5 lb, 8 oz) | 0 | |
| 2,501 to 3,000 g (5 lb, 8 oz to 6 lb, 10 oz) | 3 | |
| 3,001 to 3,500 g (6 lb, 10 oz to 7 lb, 11 oz) | 6 | |
| 3,501 to 4,000 g (7 lb, 11 oz to 8 lb, 13 oz) | 9 | |
| 4,001 to 4,500 g (8 lb, 13 oz to 9 lb, 15 oz) | 12 | |
| 4,501 to 5,000 g (9 lb, 15 oz to 11 lb, 1 oz) | 15 | |
| Oxytocin (Pitocin) used during delivery | 4 | |
| Vacuum-assisted delivery | 4 | |
| Breast and bottle feeding | 4 | |
| Exclusive breastfeeding | 5 | |
| Gestational age < 38 weeks | 5 | |
Although screening can identify infants whose TSB level will likely exceed the 95th percentile, the U.S. Preventive Services Task Force and the American Academy of Family Physicians found insufficient evidence that screening for hyperbilirubinemia is associated with improved clinical outcomes.10,11 Screening will identify infants earlier who require phototherapy, but there is no evidence that phototherapy or exchange transfusion decreases the risk of bilirubin encephalopathy.12 Universal screening increases phototherapy rates, possibly inappropriately. A large retrospective study of infants in hospitals that used universal TSB/TcB screening found that their rates of phototherapy were more than twice those of hospitals without universal screening (9.1% vs. 4.2%; P < .001), and that only 56% of the infants who received phototherapy had a TSB level in the recommended range for phototherapy.13 However, screening decreases rates of readmission for hyperbilirubinemia.14 The cost of universal TSB/TcB screening to prevent one case of kernicterus is estimated to be $5.7 million to $9.1 million.15
How Should Infants with Jaundice Be Evaluated?
Visual inspection is not an accurate method to determine bilirubin levels and often misses severe hyperbilirubinemia.16 All infants who appear jaundiced should be evaluated with a risk score or TSB/TcB measurement. The bilirubin level should be interpreted according to the infants' age in hours (Figure 15 and Figure 217 ). Further testing may be indicated depending on the infant's risk. Multiple studies have shown that TcB has a linear correlation with TSB at lower levels, but less so at higher levels.4
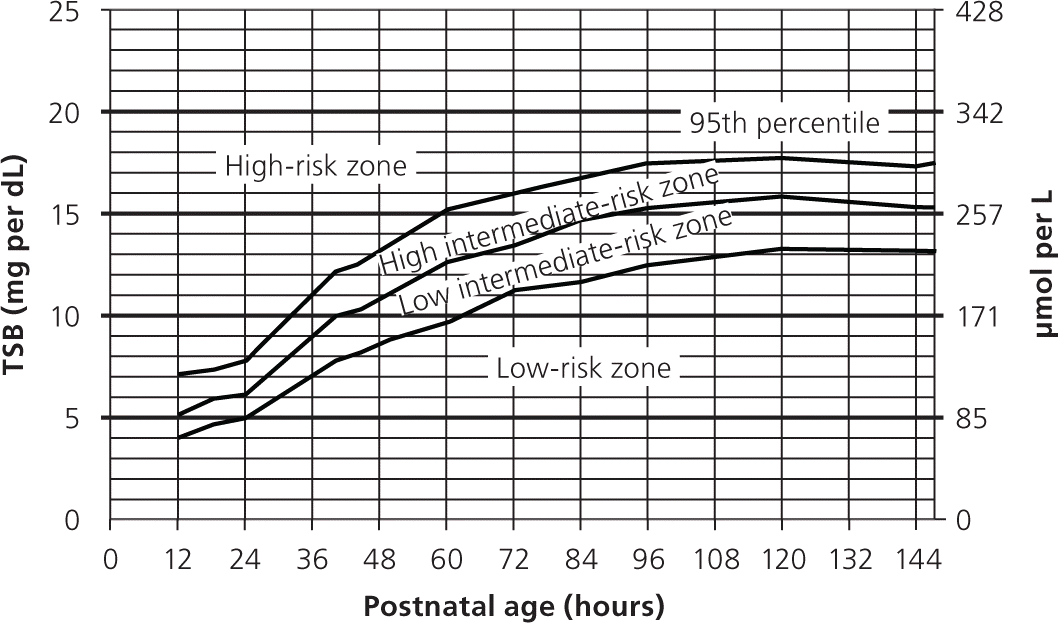
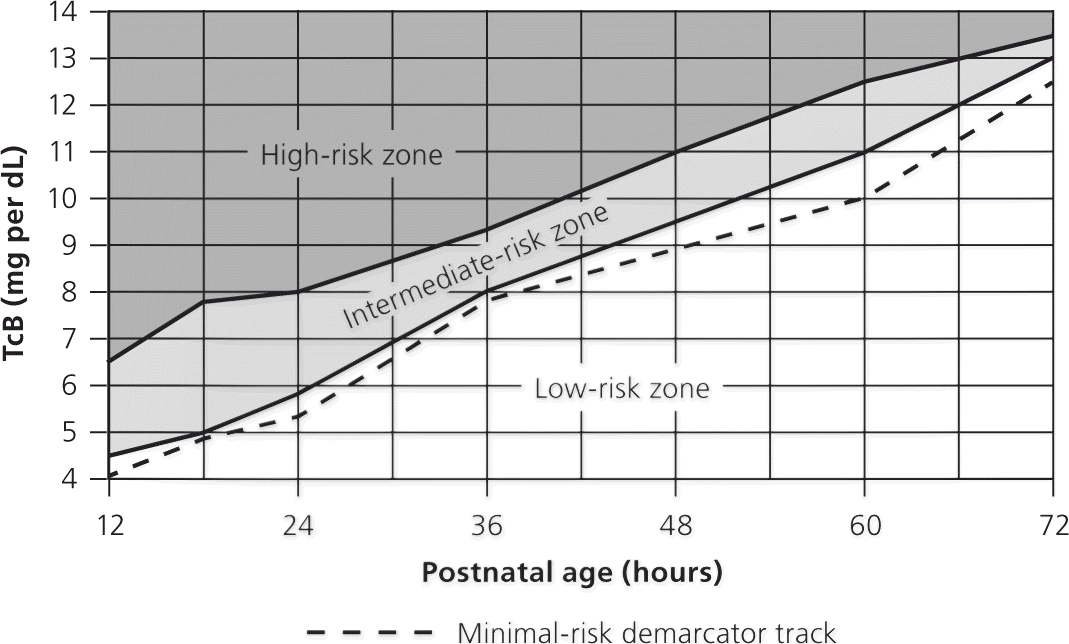
The American Academy of Pediatrics recommends the following laboratory tests for all infants with jaundice who require phototherapy: neonatal blood type, direct antibody titer or Coombs test, complete blood count and smear, and direct/conjugated bilirubin level. However, a retrospective review of 282 infants who had phototherapy and a full laboratory workup found that 88.3% had normal test results.18 Of those with abnormal results, 45% began phototherapy less than 48 hours after delivery. They also had elevated TSB levels after the initiation of phototherapy, whereas all infants with normal results had an appropriate decrease in TSB levels once phototherapy was started. These data suggest that additional tests may be necessary only if jaundice occurs in the first 48 hours of life in an infant who meets the requirements for phototherapy, or if the infant is not responding appropriately to phototherapy.
How Effective Is Treatment for Hyperbilirubinemia, and What Are the Adverse Effects?
PHOTOTHERAPY
Absorption of light through the skin converts unconjugated bilirubin into bilirubin photoproducts that are excreted in the stool and urine. The American Academy of Pediatrics has published guidelines for initiating phototherapy (Figure 3).5 Infants who were delivered at a younger gestational age or who are otherwise sick have lower thresholds for the initiation of phototherapy. The rate of decline of the TSB level after initiation of phototherapy is variable, but a 6% to 20% decrease is expected.5 In term infants without hemolysis, phototherapy can continue until the TSB level reaches 13 to 14 mg per dL (222.4 to 239.5 μmol per L). Infants do not need to be kept in the hospital to check for rebound hyperbilirubinemia, which is rare.19,20
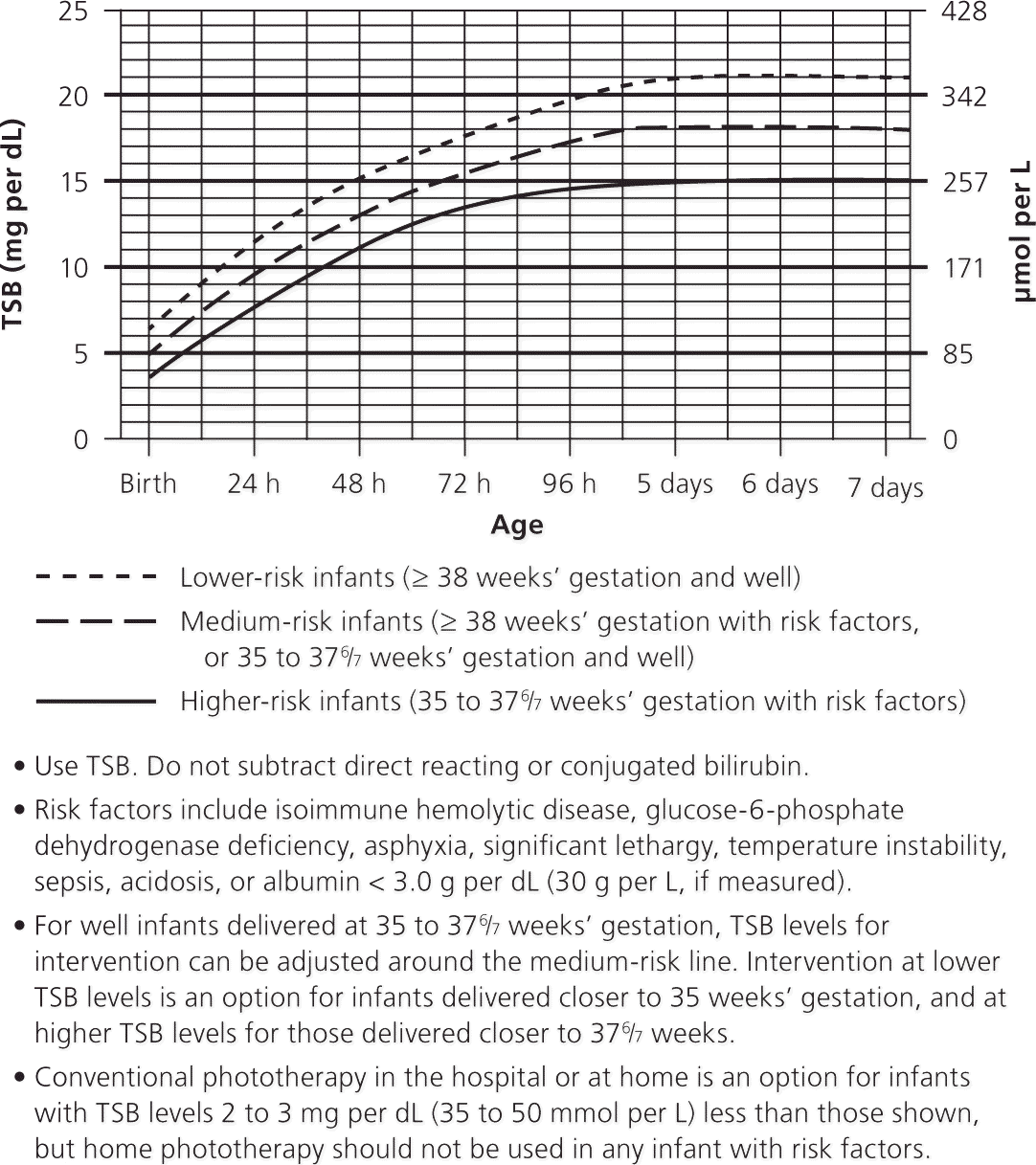
Although there is no standard protocol for phototherapy, principles include appropriate light wavelength and irradiance, and maximization of exposed body surface area. Blue to green light with wavelengths of 460 to 490 nm is the most effective in converting unconjugated bilirubin. This is considered intensive phototherapy. Infants should be naked except for their diapers to maximize the body surface area exposed to light. Types of phototherapy lights include conventional (halogen or fluorescent), light-emitting diode (LED), and fiber optic. LED and conventional lights are equally effective, with no difference in duration of phototherapy, rate of decline of the TSB level, or treatment failure.21 Standard fiber optic lights (used in home biliblankets) are not as effective as conventional lights except in preterm infants.22 However, double fiber optic lights are as effective as single conventional lights in term infants. No studies have compared home phototherapy with hospital phototherapy.
Older studies found that phototherapy results in an absolute risk reduction of 10% to 17% for preventing a TSB level greater than 20 mg per dL (number needed to treat = 5 to 10)5 and is 84% effective in preventing exchange transfusion.23 More recent evidence shows that the number needed to treat varies widely and is dependent on gestational age, sex, and age in hours.23 For example, 14 neonates need to be treated with phototherapy to prevent one exchange transfusion in a boy younger than 24 hours who was delivered at 35 weeks' gestation, but 2,176 need to be treated to prevent one in a girl older than 72 hours who was delivered at 40 weeks' gestation.
Phototherapy has short- and long-term adverse effects (Table 2).5,22,24–27 It requires physical separation of the mother and infant, blood draws, and in some cases, prolonged hospitalization. These can be emotionally distressing to parents. Studies have found that parents of infants with significant jaundice report more separation difficulties and are more likely to bring their children in for sick visits than parents of infants with similar health status.28,29 A large cohort study found that there was a higher rate of visits to subspecialists and illness visits to primary care physicians during the first 60 days of life in children who had received phototherapy (relative risk = 1.07; 95% confidence interval, 1.05 to 1.10).30
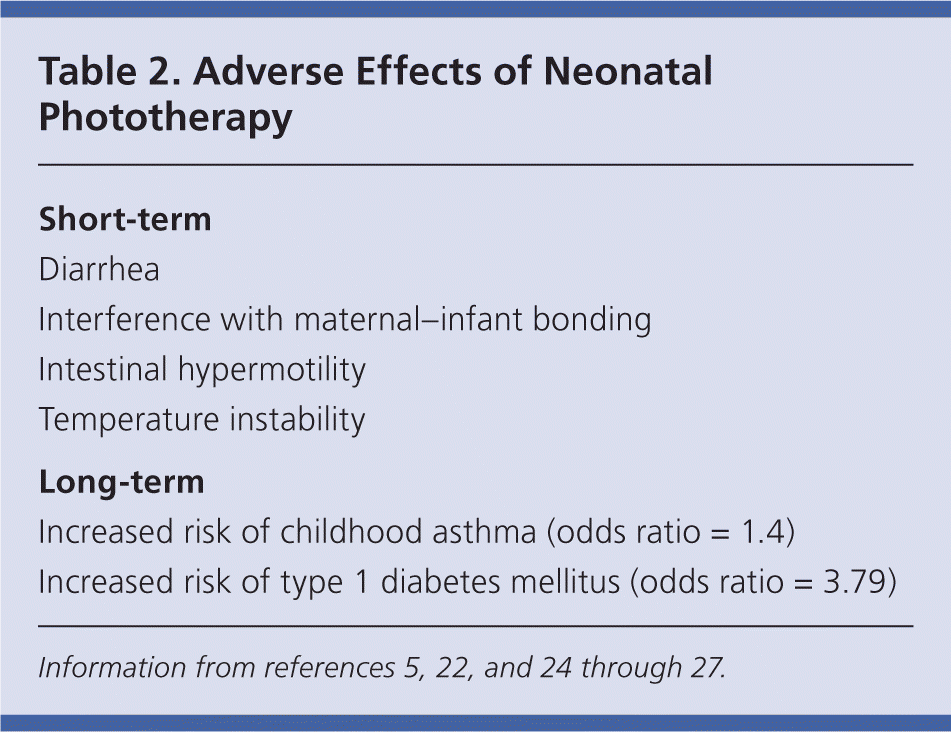
| Short-term |
| Diarrhea |
| Interference with maternal–infant bonding |
| Intestinal hypermotility |
| Temperature instability |
| Long-term |
| Increased risk of childhood asthma (odds ratio = 1.4) |
| Increased risk of type 1 diabetes mellitus (odds ratio = 3.79) |
EXCHANGE TRANSFUSION
Although phototherapy is effective in the treatment of hyperbilirubinemia, exchange transfusion is occasionally indicated. A nomogram for exchange transfusion based on TSB levels is available.5 Exchange transfusion should be performed in infants with TSB levels in the range indicated by the nomogram, with TSB levels of 25 mg per dL (427.6 μmol per L) or greater, and with jaundice and signs of acute bilirubin encephalopathy.5 Mortality within six hours of exchange transfusion in term infants without hemolysis is three or four per 1,000 infants.6 About 5% of infants who undergo exchange transfusion have blood-related or cardiorespiratory complications, metabolic derangements, or complications from central line placement.5
Should Breastfeeding Be Modified for Infants with Jaundice?
Breastfed infants are three times more likely to have a TSB level greater than 12 mg per dL (205.3 μmol per L) and six times more likely to have a level greater than 15 mg per dL (256.6 μmol per L).31 The exact mechanism for breastfeeding-related jaundice is unknown, but may involve decreased caloric intake, inhibition of hepatic bilirubin excretion, and increased intestinal bilirubin resorption. One study compared neonates who were exclusively breastfed with those who received supplemental formula if they had significant weight loss, and others who were formula fed.32 The results suggest that caloric deprivation—not necessarily breastfeeding—increases the risk of hyperbilirubinemia. Increasing the frequency of breastfeeding decreases the likelihood of significant hyperbilirubinemia.5 Signs of adequate intake in breast-fed infants include four to six thoroughly wet diapers per day, three to four stools per day by the fourth day of life, and a transition to seedy, mustard-colored stools by the third or fourth day of life.5
Breastfeeding women whose infants have jaundice are at increased risk of early cessation of breastfeeding. A study of 209 infants found that twice as many mothers of infants with jaundice stopped breastfeeding at one month compared with those whose infants did not have jaundice (number needed to harm = 4).28 Infants whose breastfeeding was interrupted for treatment of jaundice were more likely to not be breastfed at one month of age (number needed to harm = 4). Another study found that maternal interaction with health care professionals (e.g., breastfeeding orders, encouragement) was the strongest predictor of breastfeeding continuation for infants with jaundice.33
The American Academy of Pediatrics recommends promoting breastfeeding for infants with jaundice, assessing for the adequacy of breastfeeding, and increasing the frequency to eight to 12 times per day.5 Supplementation with formula may be considered if the infant's intake is inadequate, weight loss is excessive, the infant appears dehydrated, or the jaundice is severe. Phototherapy should be interrupted for breastfeeding unless the infant's bilirubin levels are approaching those that require exchange transfusion.
Are There Long-Term Neurodevelopmental Sequelae from Hyperbilirubinemia?
A prospective case-control study of 146 term and near-term infants with TSB levels greater than 25 mg per dL found no differences in cognitive scores, abnormal results on neurologic examinations, or neurologic diagnoses at two years of age.34 However, children who had a positive direct antibody titer had lower cognitive scores (average decrease in IQ score = 7).
A large prospective cohort study of children delivered at 35 weeks' gestation or later compared those with TSB levels greater than 13.5 mg per dL (230.9 μmol per L) with those who have levels less than 13.5 mg per dL.35 At two years' follow-up, there were no significant differences in rates of cerebral palsy, deafness, developmental delay, or visual abnormalities. The cohort with TSB levels greater than 19 mg per dL (325.0 μmol per L) had an increased risk of attention deficit disorder (relative risk = 1.9; 95% confidence interval, 1.1 to 3.3). Four high-quality studies with follow-up of 6.5 to 17 years showed no relationship between hyperbilirubinemia and lower IQ scores.5
Data Sources: Ovid Medline, the Cochrane Database of Systematic Reviews, Essential Evidence Plus, the U.S. Preventive Services Task Force, and the Canadian Task Force on Preventive Health Care were searched using the key terms neonatal hyperbilirubinemia, neonatal jaundice, maternal experience, and breastfeeding and jaundice. Search dates: January 2012 to February 2014.
