
Am Fam Physician. 2014;90(1):26-32
Related editorial: Beyond Analgesia in Palliative Care and End-of-Life Interventions
Patient information: See related handout on managing pain at the end-of-life, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Although many patients experience debilitating pain at the end of life, there are many options to improve analgesia and quality of life. Pain assessment using a validated tool, with attention to patient function and specific goals, helps tailor individual treatment plans. The World Health Organization pain ladder offers a stepwise guideline for approaching pain management. However, for many patients with terminal illness, strong opioids are necessary for efficient and effective analgesia. Equianalgesic dosing tables and expert guidelines aid in initiating, monitoring, and adjusting doses of oral and parenteral opioids. Clinicians should feel comfortable administering a repeat dose after the time to peak analgesic effect if the patient is still in pain. In patients with constant pain, using scheduled long-acting opioids may significantly improve pain control. Among pain subtypes, visceral pain management usually requires multiple drugs. Neuropathic pain responds well to adjuvant pharmacotherapies, such as anticonvulsants or antidepressants, in addition to opioids. Opioid-induced hyperalgesia can occur with any dose of an opioid, but is more common with higher doses of parenteral morphine and hydromorphone. With appropriate counseling, most patients with a history of substance abuse will comply with a pain treatment plan.
Many persons experience significant pain in the final months of life.1,2 In addition to wanting to preserve as much quality of life as possible, most patients express a preference to die outside of institutional settings.3 A key element to achieving these goals is adequate pain control. Despite advances in understanding pain physiology and available pharmacotherapies, many patients with terminal illnesses, such as cancer, report untreated or undertreated pain.4
| Clinical recommendation | Evidence rating | References | Comment |
|---|---|---|---|
| Pain should be assessed regularly in all patients with terminal illness, including those with cognitive impairment. | C | 5, 7 | Recommendation from expert consensus and systematic review |
| In patients with constant pain that responds to opioids, scheduling opioids with adequate breakthrough doses provides optimal analgesia. | C | 19, 21, 29 | Recommendations from expert consensus, systematic review, and low-quality randomized controlled trials |
| When patients develop opioid tolerance, rotating to an alternative opioid may improve analgesia. | B | 33 | Systematic review of uncontrolled prospective trials and case reports |
| Tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and gabapentinoids are first-line therapies for neuropathic pain. Opioids are also effective. | A | 42–45 | Systematic reviews of prospective randomized controlled trials |
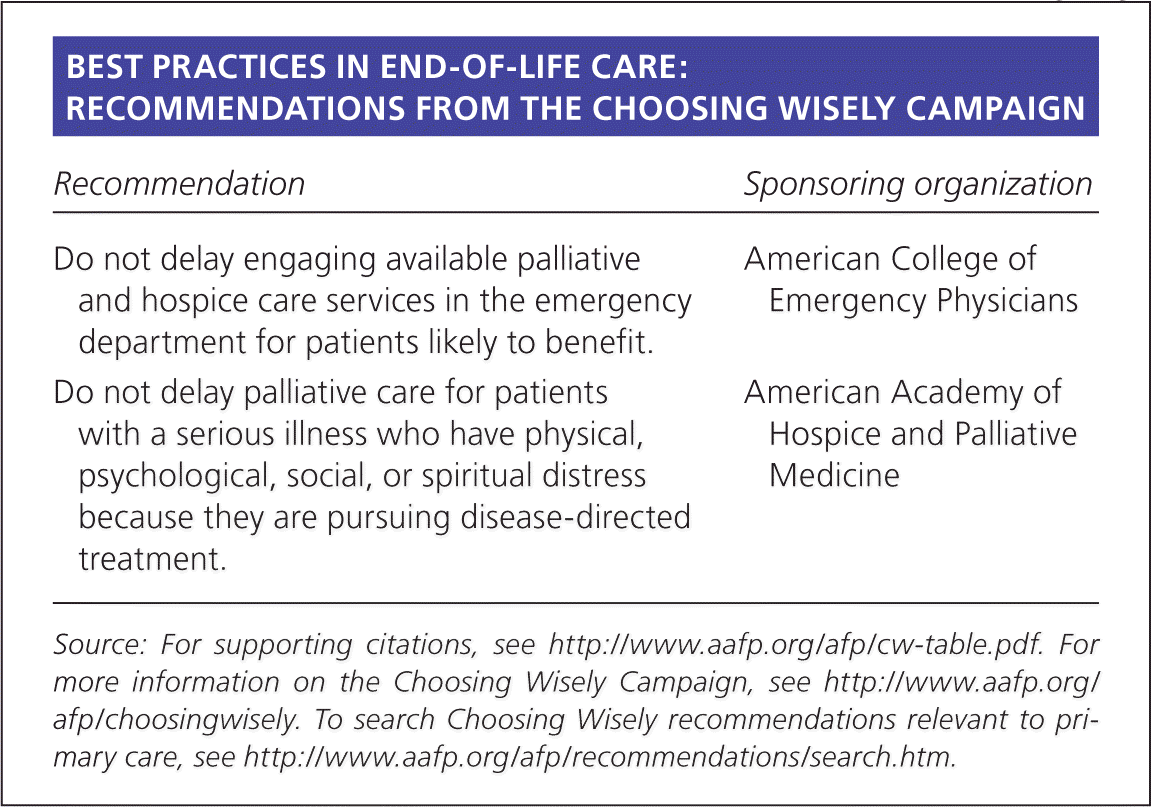
| Recommendation | Sponsoring organization |
|---|---|
| Do not delay engaging available palliative and hospice care services in the emergency department for patients likely to benefit. | American College of Emergency Physicians |
| Do not delay palliative care for patients with a serious illness who have physical, psychological, social, or spiritual distress because they are pursuing disease-directed treatment. | American Academy of Hospice and Palliative Medicine |
Pain Assessment
Assessment of pain should include location, intensity, quality, onset, duration, and factors that exacerbate or alleviate it. It is helpful to ascertain the patient's best, worst, and average pain intensities during the previous 24 hours. Physical signs of pain, such as facial grimace, tachycardia, tachypnea, or restlessness, can be helpful, although they have poor sensitivity and specificity. Patient or caregiver logs of analgesic use and pain intensity can provide essential information about the effectiveness of current interventions.
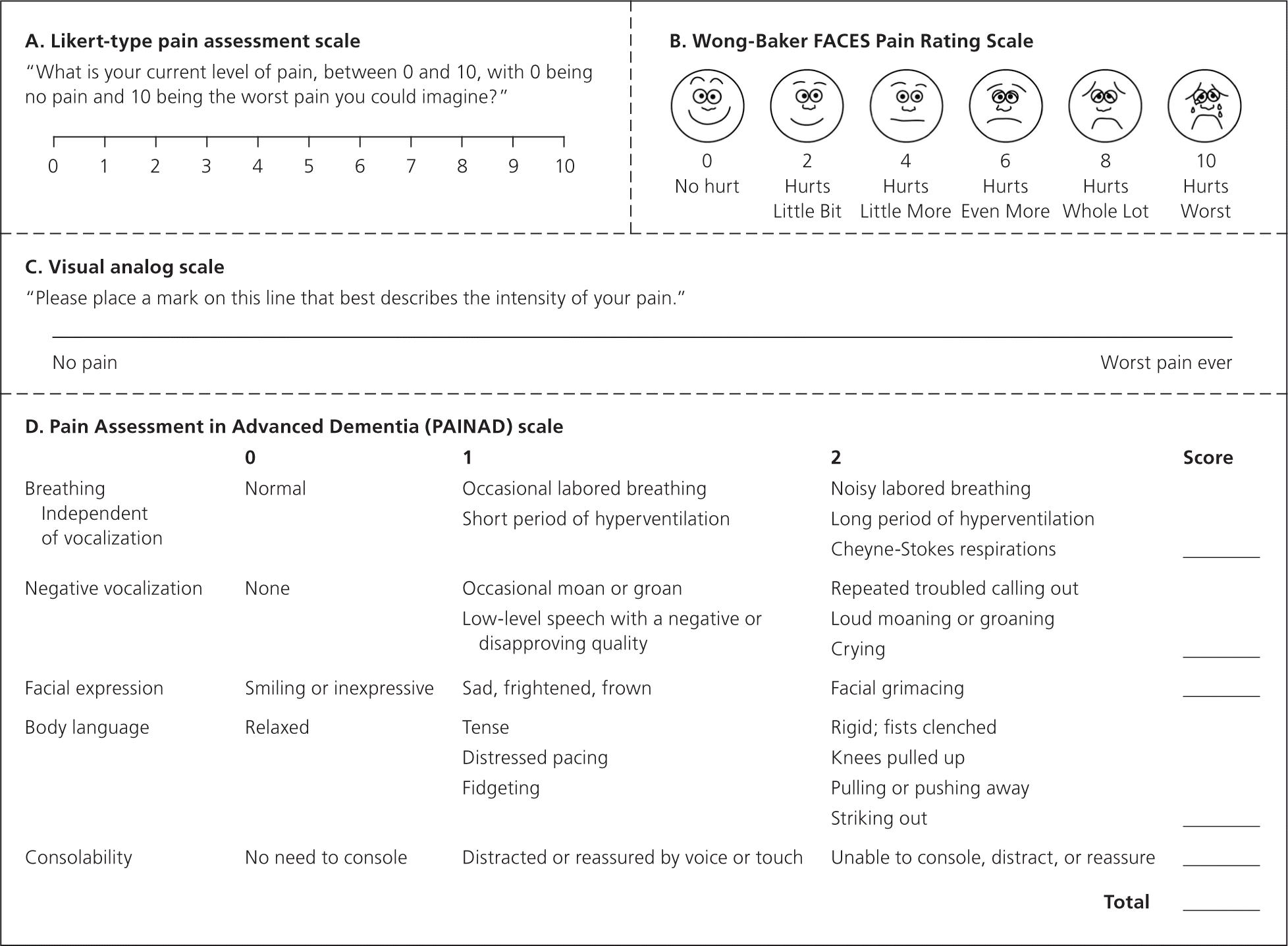
Pain should be assessed regularly. There are several pain scales and inventories to improve assessment, although none has proved better than others.5 A Likert-type scale (e.g., rating pain from 0 to 10, with 0 representing no pain and 10 representing the worst imaginable pain), the Wong-Baker FACES Pain Rating Scale, and a visual analog scale are commonly used (Figures 1A,1B,6 and 1C). Consistent use of a chosen scale allows easier assessment of the patient's pain and the effectiveness of therapies.
Pain must be assessed regularly in patients with cognitive impairment (from disease processes or pharmacotherapy) who are otherwise at risk of being undertreated for pain.7 Clinicians should first attempt direct communication because some patients with advanced dementia may be able to accurately describe pain.8 When patients cannot communicate effectively, caregivers may be interviewed. Some validated scales, such as Pain Assessment in Advanced Dementia (PAINAD; Figure 1D9 ), use objective measures to assess pain intensity and response to intervention.10 In older adults, uncontrolled pain may present as delirium.
For all patients, it is necessary to determine the connections between physical pain and function, sleep, and social relationships. Questions such as, “What does this pain keep you from doing that you would like to do?” or “How much does this pain bother you?” help focus interventions on concrete goals and quality-of-life issues. This is particularly important for patients with chronic, nonmalignant pain (e.g., lasting more than three months), for whom traditional Likert-type scales may be less useful. For example, a patient with chronic pain may report pain as 7 out of 10, but he or she may demonstrate an ability to work and socialize while not being bothered by the pain.11 Verbal descriptors often help determine underlying etiologies for somatic (e.g., aching, gnawing), visceral (e.g., cramping, shifting), or neuropathic (e.g., burning, shooting, shock-like) pain pathways.
Pain Management
FIRST-LINE AGENTS
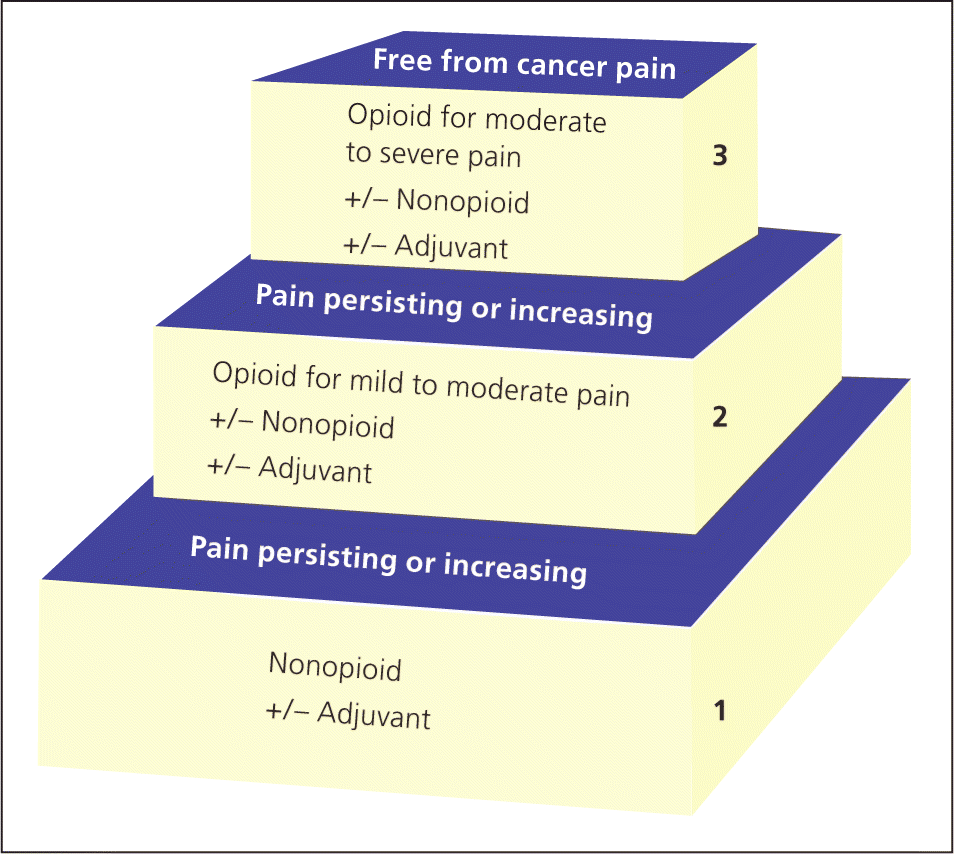
The World Health Organization cancer pain ladder provides a helpful starting point for achieving effective pain management12 (Figure 213 ). Clinicians should begin with nonopioid analgesics (e.g., acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), and gradually progress to more potent analgesics until pain is relieved. Some versions of the ladder include a fourth step for interventional procedures, such as nerve blocks or epidural infusions.14 Clinicians should be mindful that although these guidelines do promote better analgesia, strictly following the ladder may inappropriately delay adequate pain control.15 Many patients with terminal illnesses require immediate opioid therapy or have contraindications to common nonopioid analgesics, such as NSAIDs.16
Acetaminophen is useful as a primary analgesic, or in combination with other drugs, for treating mild to moderate pain. Dosages in healthy persons should be limited to no more than 4,000 mg every 24 hours to reduce the risk of hepatotoxicity. Accordingly, the U.S. Food and Drug Administration (FDA) has limited the amount of acetaminophen in all opioid combination products to 325 mg per tablet.17 Lower dosages (up to 2,000 mg every 24 hours) may be used in patients with significant liver disease.18 In patients with severe pain requiring moderate to high dosages of opioids, acetaminophen provides limited additional analgesia.19
If not contraindicated because of gastrointestinal, renal, or cardiovascular disease, NSAIDs can relieve mild to moderate pain, particularly of somatic origin (e.g., bone, muscle, skin). In certain situations, such as with pain from bony cancer metastases, NSAIDs can be a helpful adjuvant for opioid therapies. Adjuvant analgesics are defined as drugs with a primary indication other than pain that have analgesic properties in some painful conditions. Patients who are prescribed NSAIDs for more than one week should also take a proton pump inhibitor. In patients at the end of life, there are no data supporting the superiority of specific NSAIDs (including cyclooxygenase-2 inhibitors) for analgesia or for reduced adverse effects, such as gastrointestinal bleeding. NSAIDs should be selected based on dosing schedule (to maximize adherence), formulation (e.g., tablet, oral liquid, transdermal patch), cost, or route of administration (e.g., intravenous or intramuscular, oral, rectal).20
OPIOID THERAPIES
For most patients with terminal illness, opioid therapies provide the greatest analgesic relief. However, concerns about addiction or respiratory depression inappropriately limit use of opioids in these patients. Specifically, sedation (ranging from full consciousness to complete loss of consciousness) typically precedes respiratory depression.21,22 To enhance patient safety, clinicians must identify advancing sedation before it is compounded by continued opioid administration that leads to clinically significant respiratory depression.
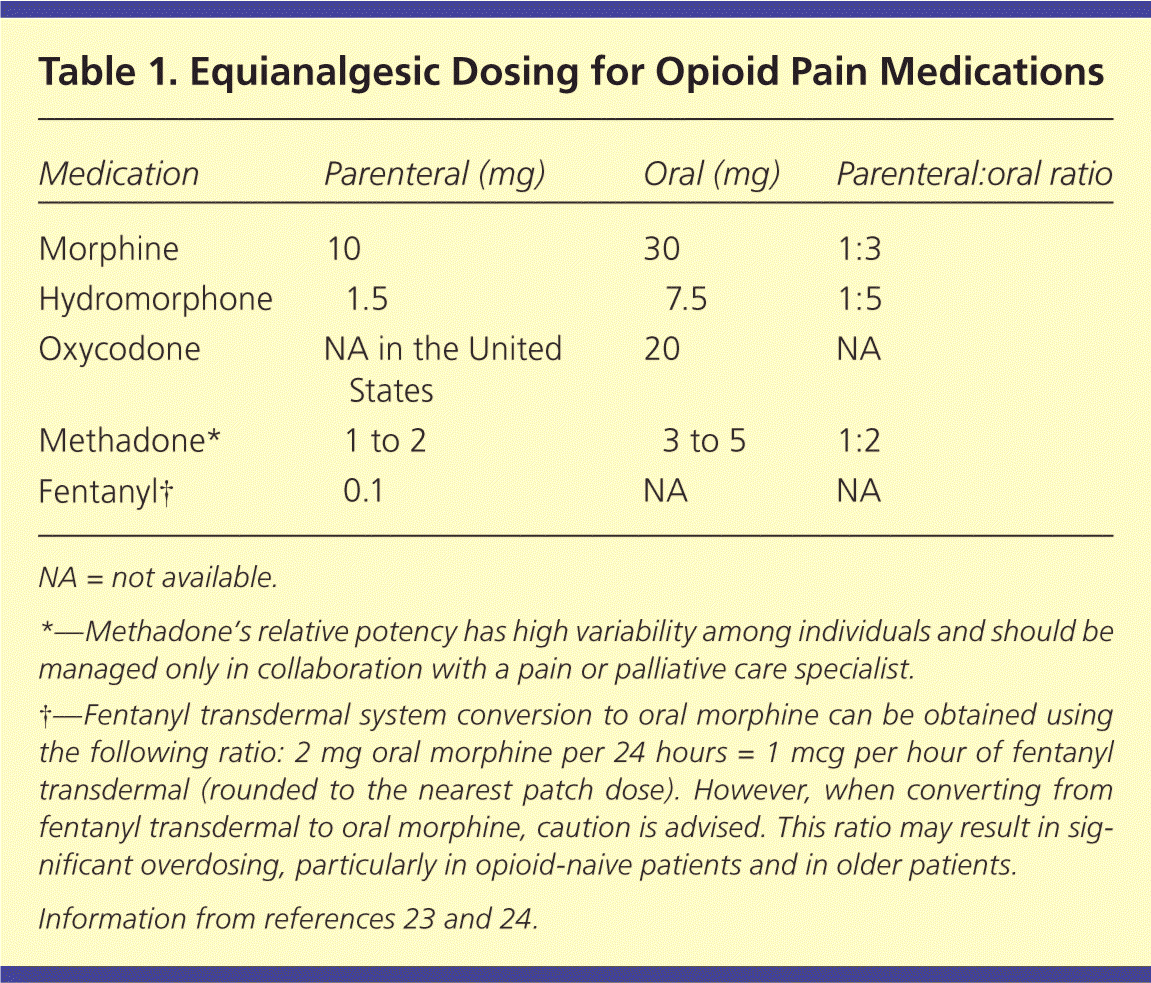
Table 1 presents equianalgesic dosing of commonly used opioid medications.23,24 It is important to be aware of time to peak analgesia and drug metabolism.25 In general, immediate-release oral opioids (e.g., morphine, oxycodone [Roxicodone], hydromorphone [Dilaudid]) reach their peak analgesic effect about one hour after administration. For intravenous opioids, the peak effect occurs after about 10 minutes. For opioids administered intramuscularly or subcutaneously, the time varies, but usually is between 20 and 30 minutes.26 Rather than waiting the length of the opioid half-life, clinicians should feel comfortable administering a repeat dose after the time to peak analgesic effect if the patient is still in pain. For example, if a patient with breakthrough pain takes 5 mg of immediate-release oxycodone at 12 p.m. but does not feel adequate relief by 1 p.m., he or she may repeat a dose at that time (i.e., time to peak effect) rather than waiting in pain until 4 p.m. (i.e., the drug's half-life). Newer opioid preparations, such as transmucosal fentanyl, aim to shorten this time frame. Administering repeat doses before the time to peak effect risks causing sedation. However, if pain remains uncontrolled after successive doses of a short-acting opioid, the dose may be increased by 25% to 50% for moderate pain or 50% to 100% for severe pain. The liver and kidneys metabolize different opioids in varying degrees; understanding relative contraindications to specific drugs promotes safety without compromising analgesia (Table 227 ).
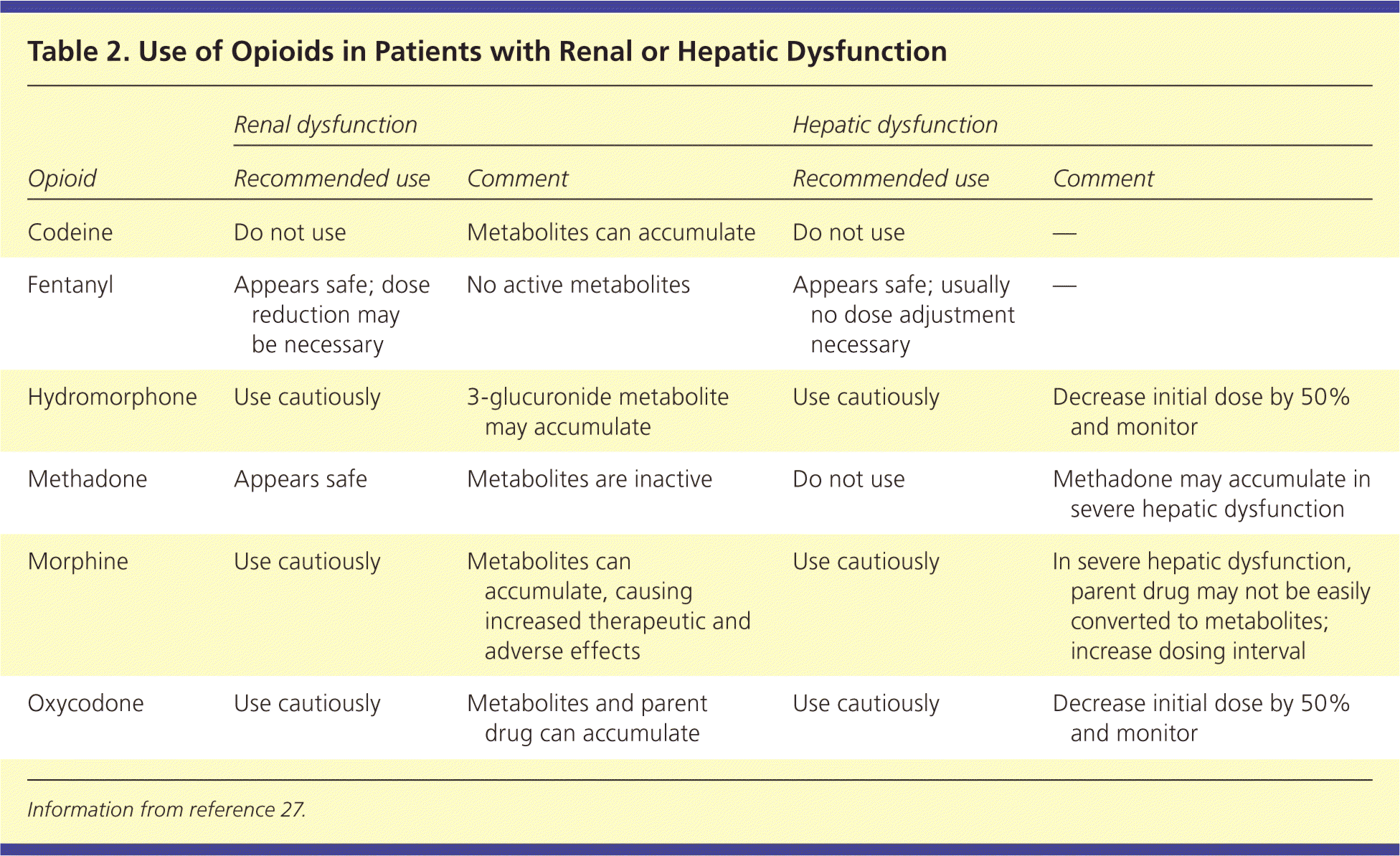
| Opioid | Renal dysfunction | Hepatic dysfunction | ||
|---|---|---|---|---|
| Recommended use | Comment | Recommended use | Comment | |
| Codeine | Do not use | Metabolites can accumulate | Do not use | — |
| Fentanyl | Appears safe; dose reduction may be necessary | No active metabolites | Appears safe; usually no dose adjustment necessary | — |
| Hydromorphone | Use cautiously | 3-glucuronide metabolite may accumulate | Use cautiously | Decrease initial dose by 50% and monitor |
| Methadone | Appears safe | Metabolites are inactive | Do not use | Methadone may accumulate in severe hepatic dysfunction |
| Morphine | Use cautiously | Metabolites can accumulate, causing increased therapeutic and adverse effects | Use cautiously | In severe hepatic dysfunction, parent drug may not be easily converted to metabolites; increase dosing interval |
| Oxycodone | Use cautiously | Metabolites and parent drug can accumulate | Use cautiously | Decrease initial dose by 50% and monitor |
Patients who consistently require multiple daily doses of short-acting opioids may benefit from a scheduled long-acting opioid. This modification can be made by totaling the patient's daily short-acting opioid use and converting 50% to 75% of that total into a long-acting drug.28 After the long-acting opioid approximates a steady state dose (about 20 to 24 hours for long-acting morphine and controlled-release oxycodone [Oxycontin], or 72 hours for transdermal fentanyl), the dose may be titrated upward using the same process. In patients with constant pain, using scheduled opioids, rather than just dosing as needed, may substantially improve pain control.19,21,29
Breakthrough pain describes periodic pain crescendos that occur despite scheduled analgesics. To manage breakthrough pain after initiation of a long-acting drug, the short-acting opioid should be available as needed. A breakthrough dose of 10% to 20% of the total daily dosage of a long-acting opioid is a reasonable starting point, although experts suggest the best dosing is determined by individual titration.30 Only oral transmucosal fentanyl has been prospectively studied for breakthrough pain; according to the FDA, it should be reserved for patients taking at least the equivalent of 60 mg of a long-acting morphine per day.31,32
Breakthrough pain is often idiopathic. However, when a known etiology (e.g., physical activity) can be identified, patients may be counseled to take their short-acting opioid ahead of time to allow the drug to reach peak analgesic effect. Management of breakthrough pain should facilitate function and social interaction. For commonly used oral opioids (i.e., morphine, oxycodone, hydrocodone, and hydromorphone), breakthrough doses should be available at least every four hours, or as often as every hour, as needed.
Patients who are unable to take opioids enterally or who require significant doses of opioids may benefit from a patient-controlled analgesia pump. The pump provides a continuous infusion programmed at an hourly basal rate. A breakthrough dose administered through a patient-controlled button is typically calculated at 50% to 100% of the hourly rate, according to the time to peak analgesic effect. When sequential doses of opioids (oral or parenteral) are administered in excess, patients experience sedation before respiratory depression. A sedated patient with a patient-controlled analgesia pump should be promptly evaluated.
There are several possible reasons why opioids may need to be rotated (e.g., cognitive decline, impaired swallowing, organ dysfunction).33,34 Because of incomplete cross-tolerance, dosing of a new opioid initially should be reduced by 25% to 50% of the newly calculated dose and then titrated to optimal analgesia.
Finally, use of alternative opioid formulations and routes of administration can enhance pain management, particularly in the outpatient setting. Patients with dysphagia often benefit from concentrated opioid elixirs; morphine, hydromorphone, oxycodone, and methadone are each produced in elixir form. Fentanyl is now available in oral transmucosal and intranasal preparations. Opioids also may be compounded into topical creams or gels for painful skin ulcers.35 Compounded transdermal gels can deliver short-acting opioids.36 Although not approved by the FDA, any opioid tablet or capsule may be administered rectally, with an initial dose similar to the oral route and with close follow-up to determine an optimal dosing schedule.37 Therapies that may be administered rectally include:
Anticonvulsants (e.g., carbamazepine [Tegretol], lamotrigine [Lamictal], phenytoin [Dilantin], valproic acid [Depakene])
Corticosteroids (e.g., dexamethasone, hydrocortisone, prednisolone)
NSAIDs (e.g., aspirin, diclofenac, ibuprofen, indomethacin [Indocin], naproxen [Naprosyn])
Opioids (e.g., codeine, hydromorphone, methadone, morphine, oxycodone, tramadol [Ultram]).38
Hydromorphone, morphine, and indomethacin are commercially available as a suppository or enema.
VISCERAL PAIN
Visceral pain, often described as squeezing, cramping, or pressure-like, is experienced by 40% of the general population and by 28% of patients with intra-abdominal metastases or who have undergone cancer treatment.39 Sources of cancer-related visceral pain include hepatic metastases, biliary obstruction, and pancreatitis, as well as pancreatic tumors and small bowel or colon obstruction. Although opioids play a key role in controlling visceral pain, nonopioid adjuvant drugs tailored to the pain's etiology may enhance analgesia. For example, NSAIDs may help treat biliary colic or inflammatory components of visceral pain; octreotide (Sandostatin) can improve pain associated with intestinal obstruction and may help prevent hyperalgesia from visceral pain.40
NEUROPATHIC PAIN
Painful neuropathies can be controlled with nonopioid analgesics, including gabapentinoids, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors (all first-line therapies), as well as anticonvulsants and sodium channel blocking antiarrhythmics.41,42 Continuous dysesthesias (e.g., pins and needles) may respond well to tricyclic antidepressants or serotonin-norepinephrine reuptake inhibitors. Lancinating pain (e.g., shock, shooting) may respond better to gabapentinoids (i.e., gabapentin [Neurontin] and pregabalin [Lyrica]). Gabapentin doses are usually titrated every three to five days, whereas pregabalin can be titrated to maximum doses within a week.43 Patients may require multiple medications.44 During titration, opioids or tramadol may provide more immediate relief of neuropathic pain.45,46 When neuropathic pain is localized and superficial, topical anesthetics (e.g., lidocaine cream, gel, or topical patch) or capsaicin (0.075% cream [Zostrix] or 8% patch [Qutenza]) may provide adequate analgesia, although capsaicin can be difficult to tolerate because of skin irritation. Patients who continue to have neuropathic pain despite treatment may benefit from consultation with a pain or palliative care specialist about other available medications or interventions.
Special Considerations
METHADONE
As an analgesic, methadone is potent, inexpensive, and may be particularly effective for neuropathic pain. It is metabolized and cleared by the liver, making it an option for patients with severe renal impairment.47 However, it also has a widely variable half-life (seven to 72 hours) and bioavailability, as well as an inactive sedating metabolite, restricting its use.48,49 Because of its complex pharmacokinetics, methadone therapy should not be initiated or managed by clinicians without appropriate training.
OPIOID-INDUCED HYPERALGESIA
Opioid-induced hyperalgesia is characterized by increasing sensitivity to pain despite increased opioid dosing, often with diffuse extension of pain location and allodynia (i.e., pain from a stimulus that does not typically cause pain, such as light touch). It can occur with any dose, but is more common with higher doses of parenteral morphine and hydromorphone.50 Initial management involves reducing or eliminating the current opioid dose, if possible, or rotating to another opioid with fewer neurotoxic effects, such as fentanyl, and maximizing indicated nonopioid adjuvants. Additional management should involve a pain or palliative care specialist.
SUBSTANCE ABUSE
Clinicians must differentiate between physiologic tolerance (i.e., a higher dose is needed to reach the same analgesia), physical dependence (i.e., a withdrawal phenomenon that manifests when the drug is discontinued), psychological dependence (i.e., an overwhelming need to acquire and use the drug despite harm to self or others), and pseudoaddiction (i.e., addiction-like behaviors resulting from inadequate pain control). When counseled appropriately, most patients with a history of substance abuse comply with a pain treatment plan.51 Only when a pattern of maladaptive behaviors unfolds should clinicians rule out pseudoaddiction and consider that the patient is experiencing drug addiction (Table 352 ).
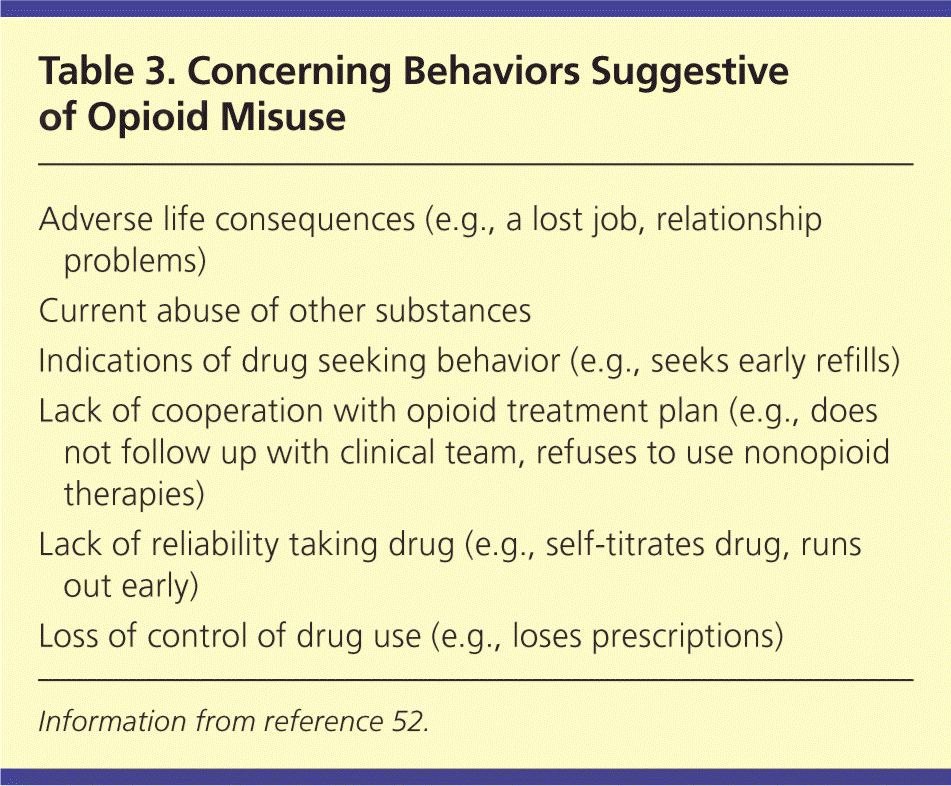
| Adverse life consequences (e.g., a lost job, relationship problems) |
| Current abuse of other substances |
| Indications of drug seeking behavior (e.g., seeks early refills) |
| Lack of cooperation with opioid treatment plan (e.g., does not follow up with clinical team, refuses to use nonopioid therapies) |
| Lack of reliability taking drug (e.g., self-titrates drug, runs out early) |
| Loss of control of drug use (e.g., loses prescriptions) |
Data Sources: A literature search was performed in PubMed and the Cochrane database using the key terms pain at end of life, breakthrough pain, opioid-induced hyperalgesia, neuropathic pain management, pain assessment in dementia, pain assessment in cognitively impaired, and other search terms. The search included meta-analyses, randomized controlled trials, prospective studies, case reports, and reviews. We also searched the Agency for Healthcare Research and Quality evidence reports, Essential Evidence Plus, the National Guideline Clearinghouse database, and UpToDate. Search dates: October through November 2012, and April 2014.
