
A more recent article on delirium in older persons is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2014;90(3):150-158
Patient information: See related handout on delirium, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Delirium is defined as an acute, fluctuating syndrome of altered attention, awareness, and cognition. It is common in older persons in the hospital and long-term care facilities and may indicate a life-threatening condition. Assessment for and prevention of delirium should occur at admission and continue throughout a hospital stay. Caregivers should be educated on preventive measures, as well as signs and symptoms of delirium and conditions that would indicate the need for immediate evaluation. Certain medications, sensory impairments, cognitive impairment, and various medical conditions are a few of the risk factors associated with delirium. Preventive interventions such as frequent reorientation, early and recurrent mobilization, pain management, adequate nutrition and hydration, reducing sensory impairments, and ensuring proper sleep patterns have all been shown to reduce the incidence of delirium, regardless of the care environment. Treatment of delirium should focus on identifying and managing the causative medical conditions, providing supportive care, preventing complications, and reinforcing preventive interventions. Pharmacologic interventions should be reserved for patients who are a threat to their own safety or the safety of others and those patients nearing death. In older persons, delirium increases the risk of functional decline, institutionalization, and death.
A 91-year-old woman with minimal English proficiency was admitted to the intensive care unit for an exacerbation of chronic obstructive pulmonary disease. Five days earlier, she had visited the emergency department for shoulder pain and was given acetaminophen with codeine. The patient's daughter reported that her mother did not comply with her chronic obstructive pulmonary disease inhaler regimen because of drowsiness brought on by the codeine. In the intensive care unit, oral food and fluids were withheld initially and the patient was given levofloxacin (Levaquin), methylprednisolone, nebulizer treatments, and ranitidine (Zantac). Overnight, she had urinary incontinence, which prompted placement of a catheter and initiation of tolterodine (Detrol) for bladder spasm. The next morning, her family arrived to find her in an anxious state, vacillating from mild fidgeting to abrupt sitting up to lying sideways on the bed. She had marked inattention and was unable to follow instructions or carry on conversation. She was confused about her whereabouts and claimed that hospital staff had attacked her.
| Clinical recommendation | Strength of recommendation | References |
|---|---|---|
| Physicians should train nursing staff, home health aides, and family members/caregivers on recognizing and treating delirium. | C | 3, 20, 21 |
| The Confusion Assessment Method is the most effective tool in identifying delirium. | C | 29 |
| Assessment for and prevention of delirium should occur at admission to the hospital and throughout the stay. | C | 3, 20, 21 |
| Multicomponent prevention methods are effective in deterring delirium episodes. | B | 20, 21, 33 |
| Antipsychotic medications should be used as a last resort in treating delirium and should not be used indiscriminately in persons with delirium who have not been properly evaluated. | A | 36–44 |
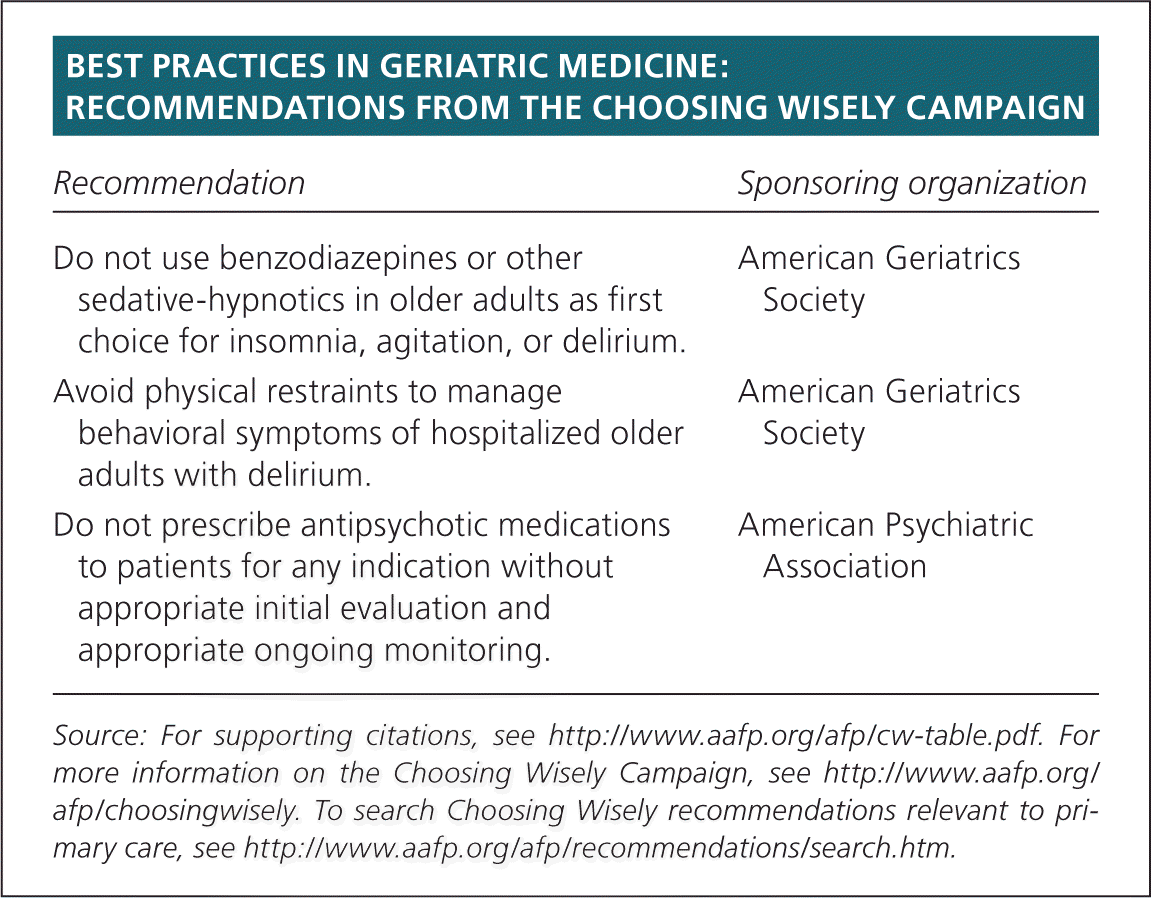
| Recommendation | Sponsoring organization |
|---|---|
| Do not use benzodiazepines or other sedative-hypnotics in older adults as first choice for insomnia, agitation, or delirium. | American Geriatrics Society |
| Avoid physical restraints to manage behavioral symptoms of hospitalized older adults with delirium. | American Geriatrics Society |
| Do not prescribe antipsychotic medications to patients for any indication without appropriate initial evaluation and appropriate ongoing monitoring. | American Psychiatric Association |
Her daughter indicated that the patient had displayed increasing “memory problems,” and was socially isolated and sedentary. The patient performed all of her activities of daily living, but had become increasingly dependent on family members for advanced tasks since a previous chronic obstructive pulmonary disease exacerbation in which the patient was intubated for 10 days.
Background
DEFINITION AND DIAGNOSTIC CRITERIA
Delirium is an acute, fluctuating syndrome of altered attention, awareness, and cognition precipitated by an underlying condition or event in vulnerable persons (Table 1).1 In practice and in the literature, it has commonly been referred to by other names, including altered mental status, acute confusional state, sundowning, encephalopathy, and acute organic brain syndrome.1
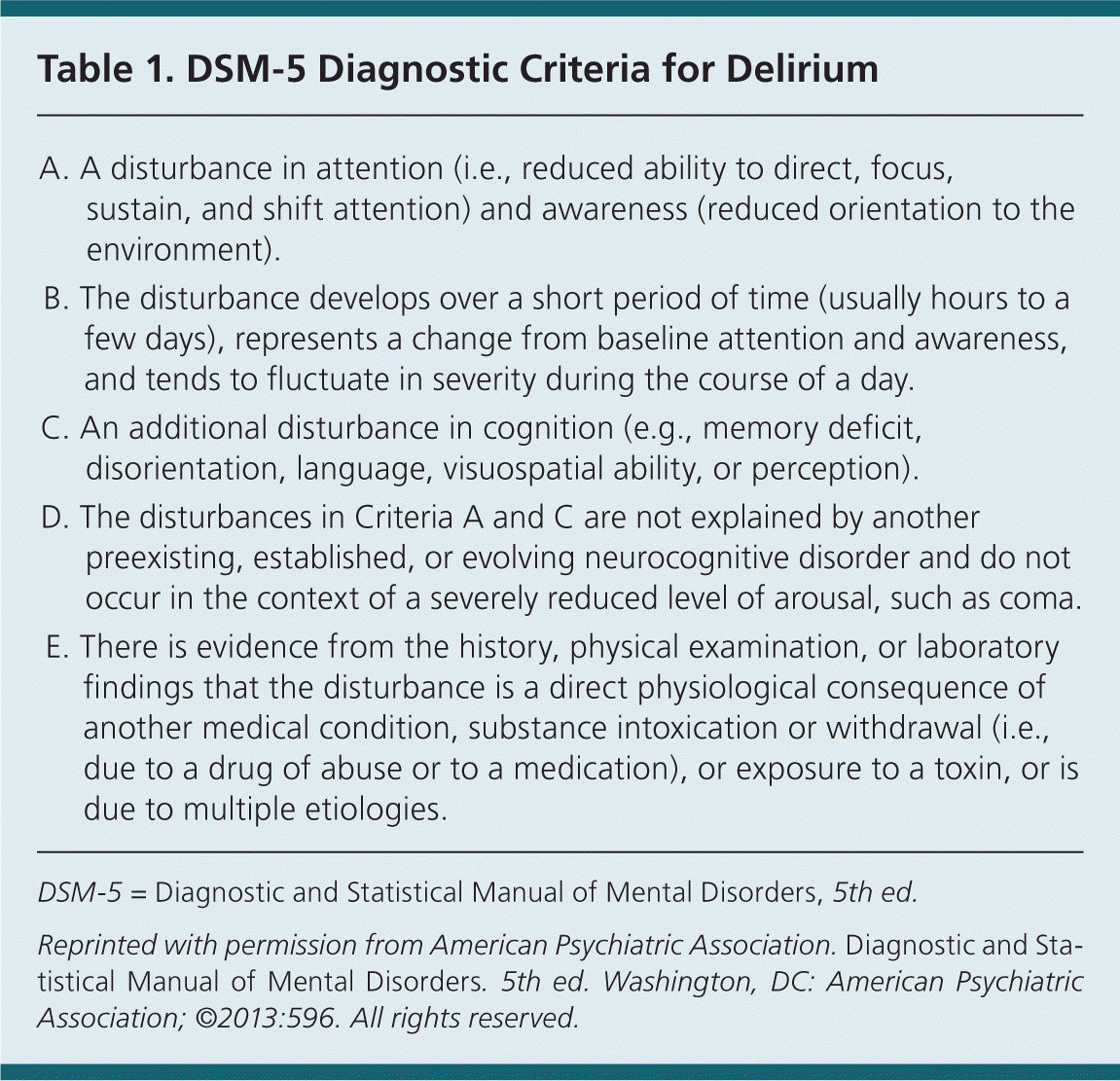
| A. A disturbance in attention (i.e., reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment). | |
| B. The disturbance develops over a short period of time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day. | |
| C. An additional disturbance in cognition (e.g., memory deficit, disorientation, language, visuospatial ability, or perception). | |
| D. The disturbances in Criteria A and C are not explained by another preexisting, established, or evolving neurocognitive disorder and do not occur in the context of a severely reduced level of arousal, such as coma. | |
| E. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct physiological consequence of another medical condition, substance intoxication or withdrawal (i.e., due to a drug of abuse or to a medication), or exposure to a toxin, or is due to multiple etiologies. |
Although delirium in general is common, many cases go unrecognized.2 This can be disconcerting to patients and caregivers, and increases the risk of functional decline and poor long-term outcomes.3,4 It was a long-held view that delirium was a completely reversible process attributable to an underlying cause. More recently, investigators recognize that a single cause is rare, and instead delirium is usually the result of many factors.2
INCIDENCE, PREVALENCE, AND SIGNIFICANCE
Delirium is common in older persons in hospitals and long-term care facilities, and it may indicate a life-threatening condition. Estimates of the prevalence of delirium vary based on the population studied, the timeframe in which delirium is assessed, and the method of assessment. Table 2 outlines the incidence and prevalence of delirium in multiple care environments.2,5–8 Immediate and long-term outcomes associated with a delirium episode include increases in the risk of falls, length of hospital stay, hospital costs, duration of mechanical ventilation, degree of cognitive impairment, functional impairment after a hospital stay, long-term care facility placement, and mortality.4,5,9–13 The estimated health care costs attributable to delirium and its complications range from $38 billion to $152 billion each year, based on 2008 calculations.14 Delirium is still missed in as many as 32% to 66% of cases.2 In many patients, global cerebral function may never return to baseline.15
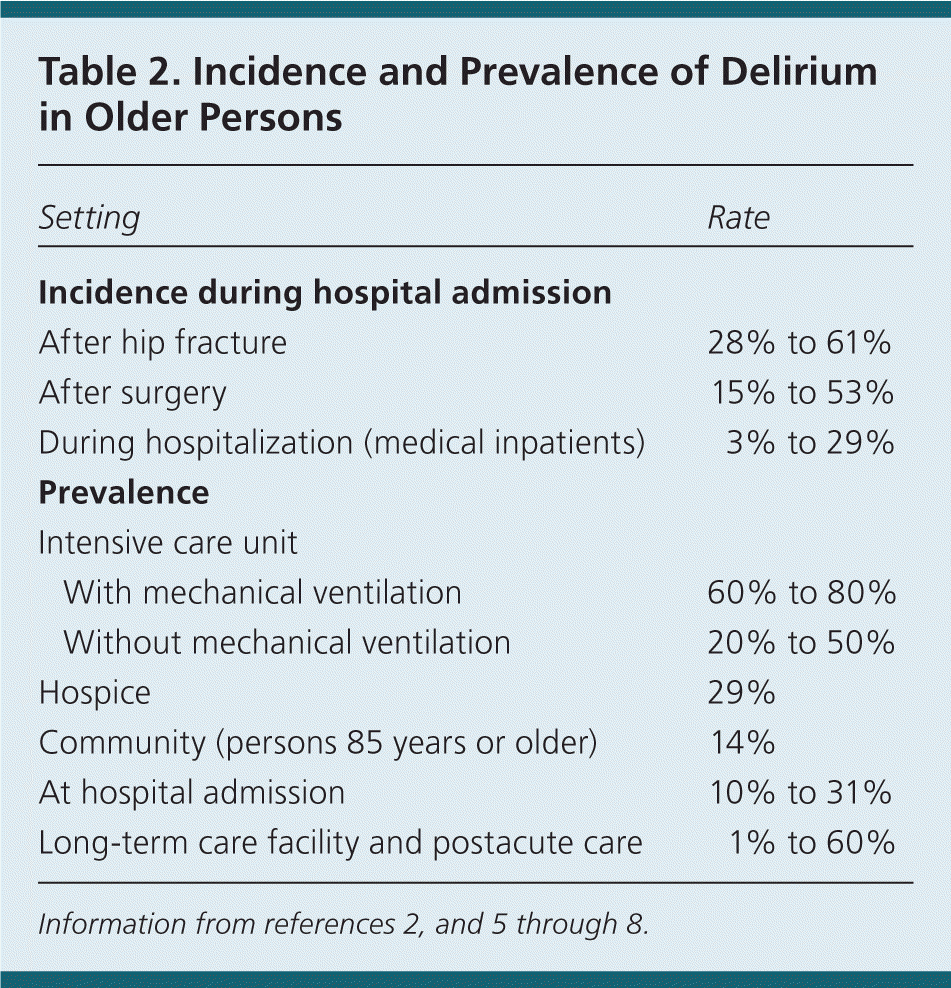
| Setting | Rate | |
|---|---|---|
| Incidence during hospital admission | ||
| After hip fracture | 28% to 61% | |
| After surgery | 15% to 53% | |
| During hospitalization (medical inpatients) | 3% to 29% | |
| Prevalence | ||
| Intensive care unit | ||
| With mechanical ventilation | 60% to 80% | |
| Without mechanical ventilation | 20% to 50% | |
| Hospice | 29% | |
| Community (persons 85 years or older) | 14% | |
| At hospital admission | 10% to 31% | |
| Long-term care facility and postacute care | 1% to 60% | |
RISK FACTORS
Assessing a patient's medical disposition, physical and cognitive impairments, and social behaviors is essential for targeting at-risk patients with prevention strategies, as well as for identifying the multiple causes associated with a single delirium episode. Delirium shares risk factors with other geriatric syndromes, such as dementia, depression, malnutrition, pressure ulcers, elder abuse, urinary incontinence, chronic pain, and falls.16 Frailty denotes a physical vulnerability and is linked to cognitive vulnerability (delirium) by uncertain pathways. It is indirectly suggested that frailty is an independent risk factor for delirium, but further research is needed.17
Risks for delirium can be divided into predisposing and precipitating factors (Table 3).2,15,18,19 Patients at high risk of delirium because of multiple or severe predisposing factors need minimal precipitators to provoke a delirium episode. Alternatively, a patient with few predisposing factors would require multiple or severe triggers to provoke delirium.2,15 Physicians should train nursing staff, home health aides, and family members/caregivers on recognizing and treating delirium.3,20,21
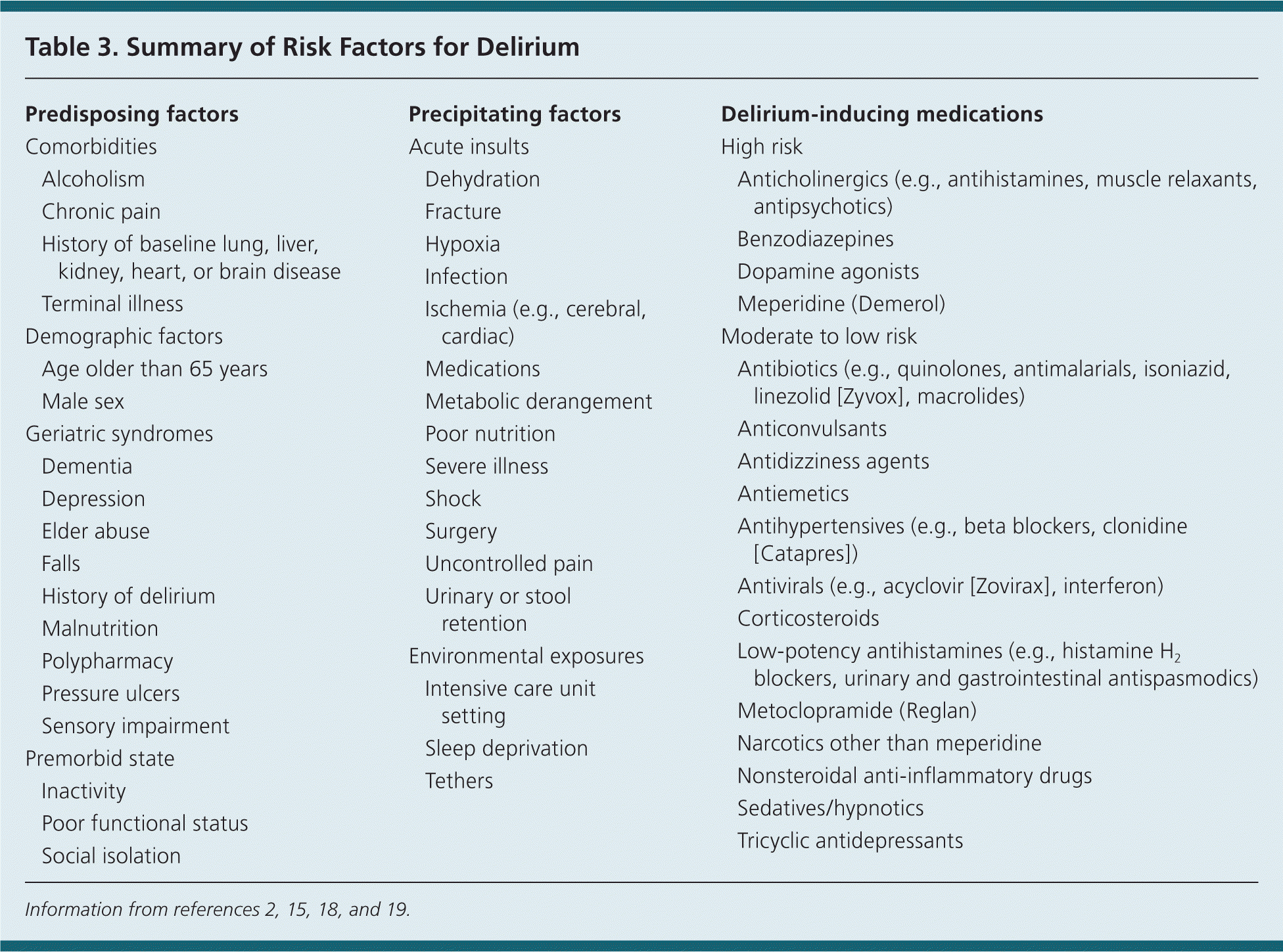
| Predisposing factors | |
| Comorbidities | |
| Alcoholism | |
| Chronic pain | |
| History of baseline lung, liver, kidney, heart, or brain disease | |
| Terminal illness | |
| Demographic factors | |
| Age older than 65 years | |
| Male sex | |
| Geriatric syndromes | |
| Dementia | |
| Depression | |
| Elder abuse | |
| Falls | |
| History of delirium | |
| Malnutrition | |
| Polypharmacy | |
| Pressure ulcers | |
| Sensory impairment | |
| Premorbid state | |
| Inactivity | |
| Poor functional status | |
| Social isolation | |
| Precipitating factors | |
| Acute insults | |
| Dehydration | |
| Fracture | |
| Hypoxia | |
| Infection | |
| Ischemia (e.g., cerebral, cardiac) | |
| Medications | |
| Metabolic derangement | |
| Poor nutrition | |
| Severe illness | |
| Shock | |
| Surgery | |
| Uncontrolled pain | |
| Urinary or stool retention | |
| Environmental exposures | |
| Intensive care unit setting | |
| Sleep deprivation | |
| Tethers | |
| Delirium-inducing medications | |
| High risk | |
| Anticholinergics (e.g., antihistamines, muscle relaxants, antipsychotics) | |
| Benzodiazepines | |
| Dopamine agonists | |
| Meperidine (Demerol) | |
| Moderate to low risk | |
| Antibiotics (e.g., quinolones, antimalarials, isoniazid, linezolid [Zyvox], macrolides) | |
| Anticonvulsants | |
| Antidizziness agents | |
| Antiemetics | |
| Antihypertensives (e.g., beta blockers, clonidine [Catapres]) | |
| Antivirals (e.g., acyclovir [Zovirax], interferon) | |
| Corticosteroids | |
| Low-potency antihistamines (e.g., histamine H2 blockers, urinary and gastrointestinal antispasmodics) | |
| Metoclopramide (Reglan) | |
| Narcotics other than meperidine | |
| Nonsteroidal anti-inflammatory drugs | |
| Sedatives/hypnotics | |
| Tricyclic antidepressants | |
Presentation in Various Settings
HOSPITAL
There are three subtypes of delirium: hypoactive, hyperactive, and mixed. The older patient commonly presents in the hypoactive form, which often goes unrecognized.18,19,22,23 Hypoactive delirium consists of at least four of the following behaviors: unawareness, decreased alertness, sparse or slow speech, lethargy, slowed movements, staring, or apathy.18,19,22,23 Alternatively, hyperactive delirium presents with at least three of the following characteristics: hypervigilance, restlessness, fast or loud speech, irritability, combativeness, impatience, swearing, singing, laughing, uncooperativeness, euphoria, anger, wandering, easy startling, fast motor responses, distractibility, tangentiality, nightmares, or persistent thoughts. The mixed subtype presents with characteristics of hypoactive and hyperactive delirium and is the most commonly diagnosed subtype.18,19,22,23
COMMUNITY
Patients recently discharged directly to home are at an increased risk of delirium, particularly if they were delirious while hospitalized.4,14,22 Patients may be discharged home in a persistent delirious state or while experiencing subsyndromal delirium, in which some but not all of the core symptoms of delirium persist.9,15 Caregivers of persons with advanced disease or dementia often witness changes in behavior that may or may not suggest delirium. These patients may experience loss of behavior control, mood fluctuations, episodes of frank psychosis, or agitation.24
LONG-TERM CARE FACILITY
Patients in long-term care facilities are at high risk of delirium because of cognitive or physical disability. The prevalence of delirium in a long-term care facility is not firmly established, ranging from 1% to 60%.15 Patients typically present with the hypoactive form of delirium in this setting.15
NEARING DEATH
Hypoactive delirium is most common in the hospice or palliative care setting; it occurs in 20% to 83% of inpatients in palliative care units.25,26 Nevertheless, delirium in patients nearing death is often misdiagnosed, usually mistaken for depression or severe fatigue.25 In patients with a terminal illness, delirium presages death within days to weeks.25,26 Recognizing delirium is vital for treatment planning and for advising family members on what to anticipate.25
Regardless of the setting, caregivers should be educated on the signs and symptoms of delirium and conditions that would indicate the need for immediate evaluation, including dramatic changes in vital signs, acute respiratory distress, chest pain, hematuria, and new-onset neurologic focal deficits.27
Predicting Delirium
Efforts to predict delirium in older hospitalized patients should be made on initial evaluation. Paying close attention to the patient's history, changes in medications, laboratory values, and physical examination findings can assist with determining a patient's risk of delirium. These predisposing and eliciting factors have been developed into a validated prognostic model (Table 4) that can identify a subset of patients at high risk of delirium during their stay.3 Although this predictive model is limited to use in medical inpatients, models have been derived for patients in alternative settings.
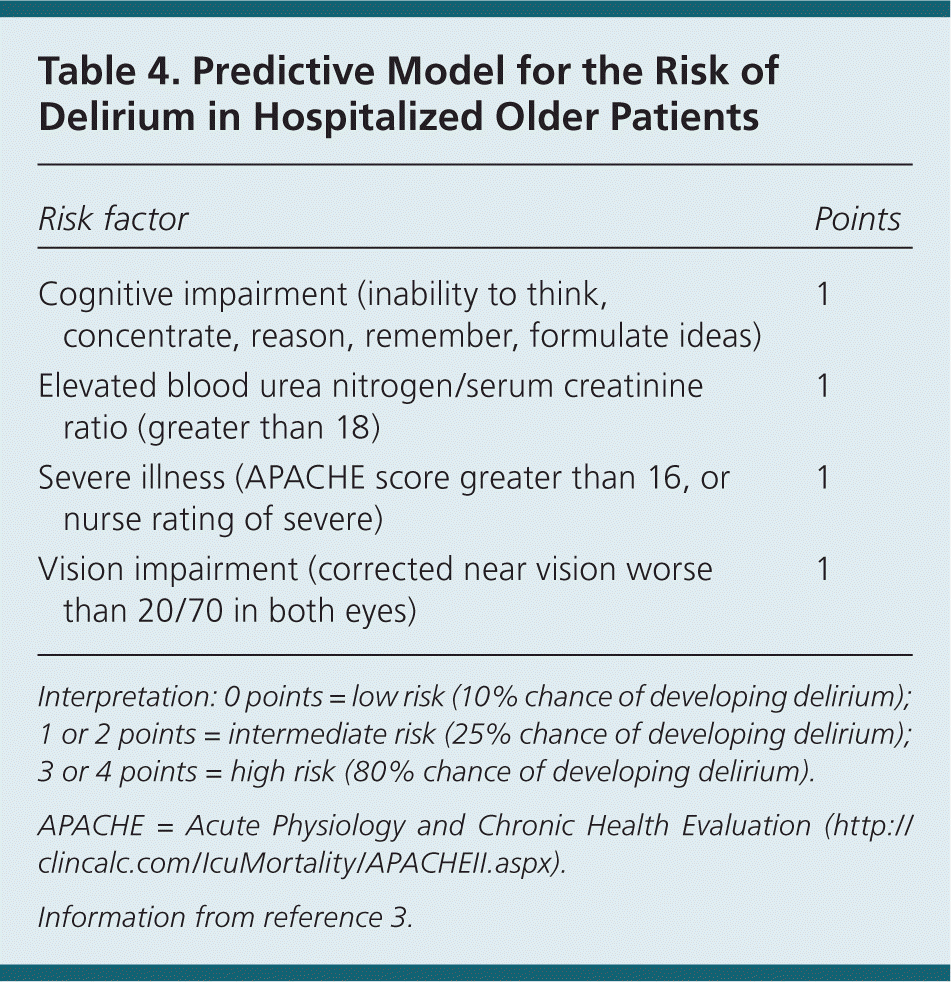
| Risk factor | Points |
|---|---|
| Cognitive impairment (inability to think, concentrate, reason, remember, formulate ideas) | 1 |
| Elevated blood urea nitrogen/serum creatinine ratio (greater than 18) | 1 |
| Severe illness (APACHE score greater than 16, or nurse rating of severe) | 1 |
| Vision impairment (corrected near vision worse than 20/70 in both eyes) | 1 |
Diagnostic Testing
All older persons presenting with delirium require a basic workup including a complete blood count, measurement of electrolyte levels, renal and liver panel, urinalysis, and electrocardiography. Evaluation should be individualized based on the patient's chief concern, medical history, current illness, and physical examination findings. Although imaging is not generally indicated, computed tomography of the head is recommended for patients presenting with new focal neurologic deficits, history of head trauma, or fever associated with encephalopathy. If seizures are suspected, or if the cause of delirium is unclear, electroencephalography should be considered. Table 5 outlines studies that can identify medical conditions that may lead to delirium.18 It is helpful if a baseline cognition assessment (e.g., Mini-Mental State Examination, Mini-Cog, Short Portable Mental Status Questionnaire) was performed and recorded before delirium occurrence.
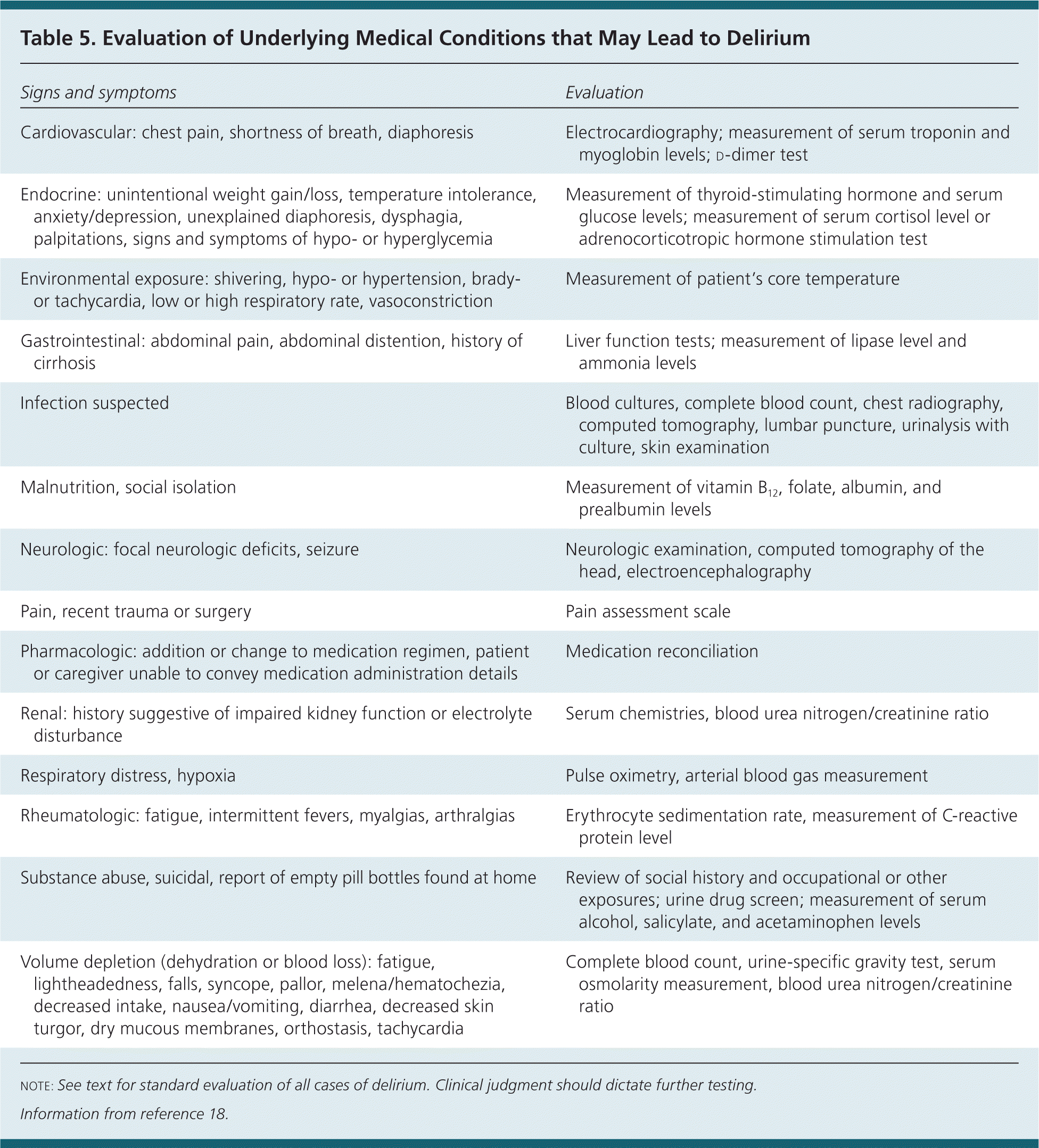
| Signs and symptoms | Evaluation |
|---|---|
| Cardiovascular: chest pain, shortness of breath, diaphoresis | Electrocardiography; measurement of serum troponin and myoglobin levels; d-dimer test |
| Endocrine: unintentional weight gain/loss, temperature intolerance, anxiety/depression, unexplained diaphoresis, dysphagia, palpitations, signs and symptoms of hypo- or hyperglycemia | Measurement of thyroid-stimulating hormone and serum glucose levels; measurement of serum cortisol level or adrenocorticotropic hormone stimulation test |
| Environmental exposure: shivering, hypo- or hypertension, brady- or tachycardia, low or high respiratory rate, vasoconstriction | Measurement of patient's core temperature |
| Gastrointestinal: abdominal pain, abdominal distention, history of cirrhosis | Liver function tests; measurement of lipase level and ammonia levels |
| Infection suspected | Blood cultures, complete blood count, chest radiography, computed tomography, lumbar puncture, urinalysis with culture, skin examination |
| Malnutrition, social isolation | Measurement of vitamin B12, folate, albumin, and prealbumin levels |
| Neurologic: focal neurologic deficits, seizure | Neurologic examination, computed tomography of the head, electroencephalography |
| Pain, recent trauma or surgery | Pain assessment scale |
| Pharmacologic: addition or change to medication regimen, patient or caregiver unable to convey medication administration details | Medication reconciliation |
| Renal: history suggestive of impaired kidney function or electrolyte disturbance | Serum chemistries, blood urea nitrogen/creatinine ratio |
| Respiratory distress, hypoxia | Pulse oximetry, arterial blood gas measurement |
| Rheumatologic: fatigue, intermittent fevers, myalgias, arthralgias | Erythrocyte sedimentation rate, measurement of C-reactive protein level |
| Substance abuse, suicidal, report of empty pill bottles found at home | Review of social history and occupational or other exposures; urine drug screen; measurement of serum alcohol, salicylate, and acetaminophen levels |
| Volume depletion (dehydration or blood loss): fatigue, lightheadedness, falls, syncope, pallor, melena/hematochezia, decreased intake, nausea/vomiting, diarrhea, decreased skin turgor, dry mucous membranes, orthostasis, tachycardia | Complete blood count, urine-specific gravity test, serum osmolarity measurement, blood urea nitrogen/creatinine ratio |
Ongoing Assessment
Identification of delirium requires ongoing reassessment during the patient's hospital stay, at home, or in a nursing facility. The Confusion Assessment Method (Table 619 ) is the most effective tool in identifying delirium, and can assist trained physicians and nursing staff in identifying delirium at admission or throughout a patient's course of illness.2,18,19 Its sensitivity ranges from 94% to 100%.28,29 Although more research is warranted, implementation of a new Confusion Assessment Method–Severity (CAM-S) may prove beneficial in determining patient response to interventions and target treatments.30
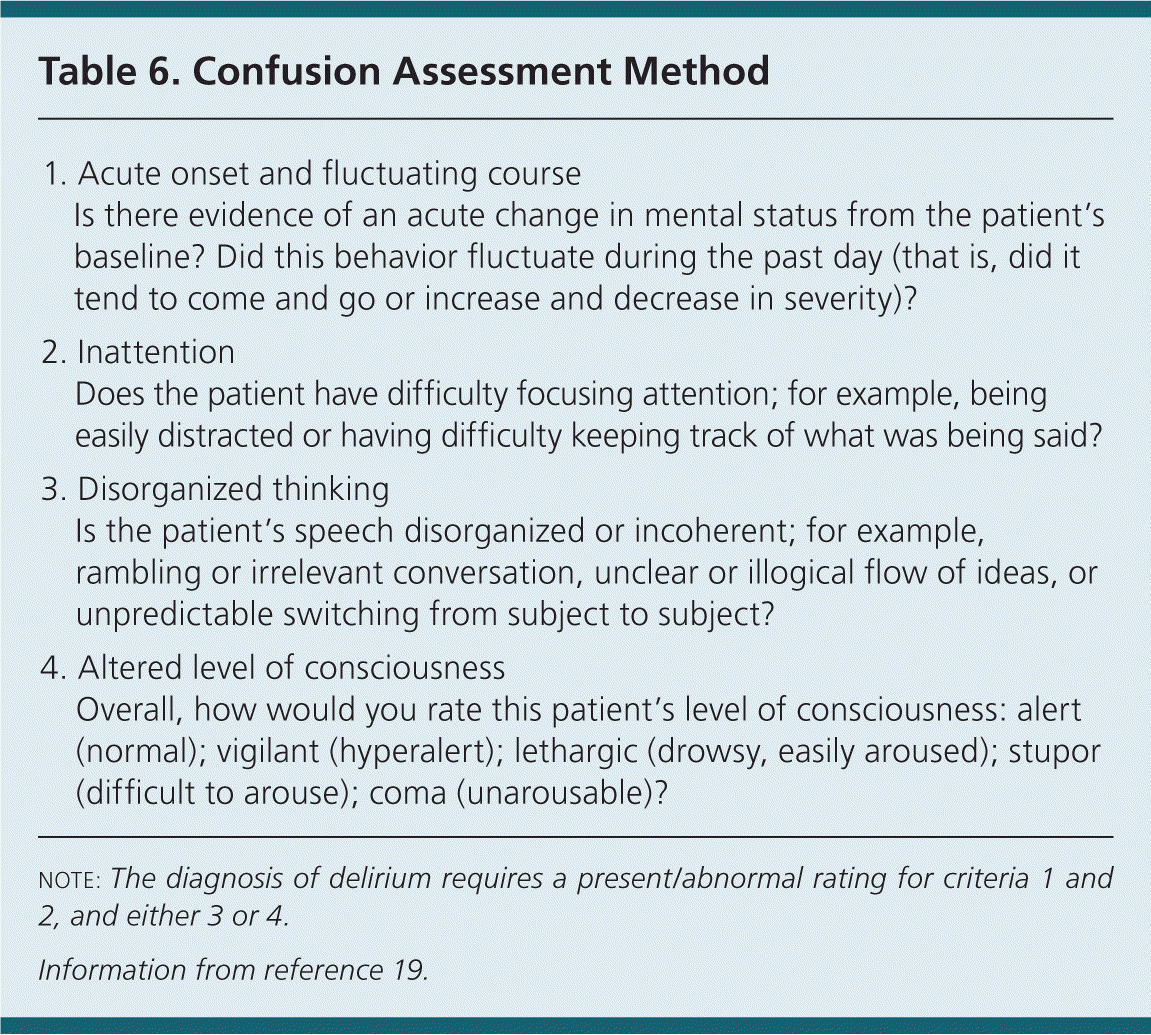
| 1. | Acute onset and fluctuating course |
| Is there evidence of an acute change in mental status from the patient's baseline? Did this behavior fluctuate during the past day (that is, did it tend to come and go or increase and decrease in severity)? | |
| 2. | Inattention |
| Does the patient have difficulty focusing attention; for example, being easily distracted or having difficulty keeping track of what was being said? | |
| 3. | Disorganized thinking |
| Is the patient's speech disorganized or incoherent; for example, rambling or irrelevant conversation, unclear or illogical flow of ideas, or unpredictable switching from subject to subject? | |
| 4. | Altered level of consciousness |
| Overall, how would you rate this patient's level of consciousness: alert (normal); vigilant (hyperalert); lethargic (drowsy, easily aroused); stupor (difficult to arouse); coma (unarousable)? |
Prevention
Assessment for and prevention of delirium should occur at admission to the hospital and throughout the stay.3,20,21 Prevention efforts targeting persons at risk may decrease delirium incidence, hospital costs, and associated poor outcomes.20,31,32 Studies have demonstrated that a multicomponent nonpharmacologic approach is highly effective and reduces the number and duration of episodes of delirium.20,21,33 One such intervention, known as the Hospital Elder Life Program, is available at http://www.hospitalelderlifeprogram.org. This intervention has been shown to reduce the number of patients with functional decline32 and placement in long-term care facilities.34 Inpatient consultation focusing on geriatric syndromes has also been shown to decrease delirium incidence.33 These interventions reduce the development of delirium, but have no effect on duration of delirium when it develops, implying that prevention strategies should be emphasized in at-risk patients.
Nonpharmacologic prevention strategies consist of orientation and therapeutic activities, early and recurrent mobilization, minimizing the use of psychoactive medications, promoting normal sleep-wake cycles, providing easy access to adaptive equipment for sensory impairment (e.g., glasses, hearing aids), and preventing dehydration.15 Orientation activities should include encouraging familiar visitors, minimizing changes in nursing staff, and ensuring that functional clocks and calendars are easily visualized.15,18 All caretakers should be educated on preventive approaches and encouraged to implement them.21 The Hospital Elder Life Program has also developed the Family Confusion Assessment Method, a validated screening tool that can be used by trained family members to detect delirium (http://hospitalelderlifeprogram.org/private/famcam-disclaimer.php?pageid=01.09.00). This tool has a demonstrated sensitivity of 86% and a specificity of 98% in one study of 58 caregivers.35
Treatment in Various Settings
HOSPITAL
Once delirium is diagnosed in an inpatient setting, it is important to identify and treat the underlying causes. After the causative factors are addressed, focus should shift to nonpharmacologic measures (Table 718,20,32,34 ), providing supportive care, and preventing complications.15,18 Although narcotics carry a risk of provoking delirium, guidelines recommend addressing uncontrolled pain through a pain management plan that may include a narcotic regimen.18
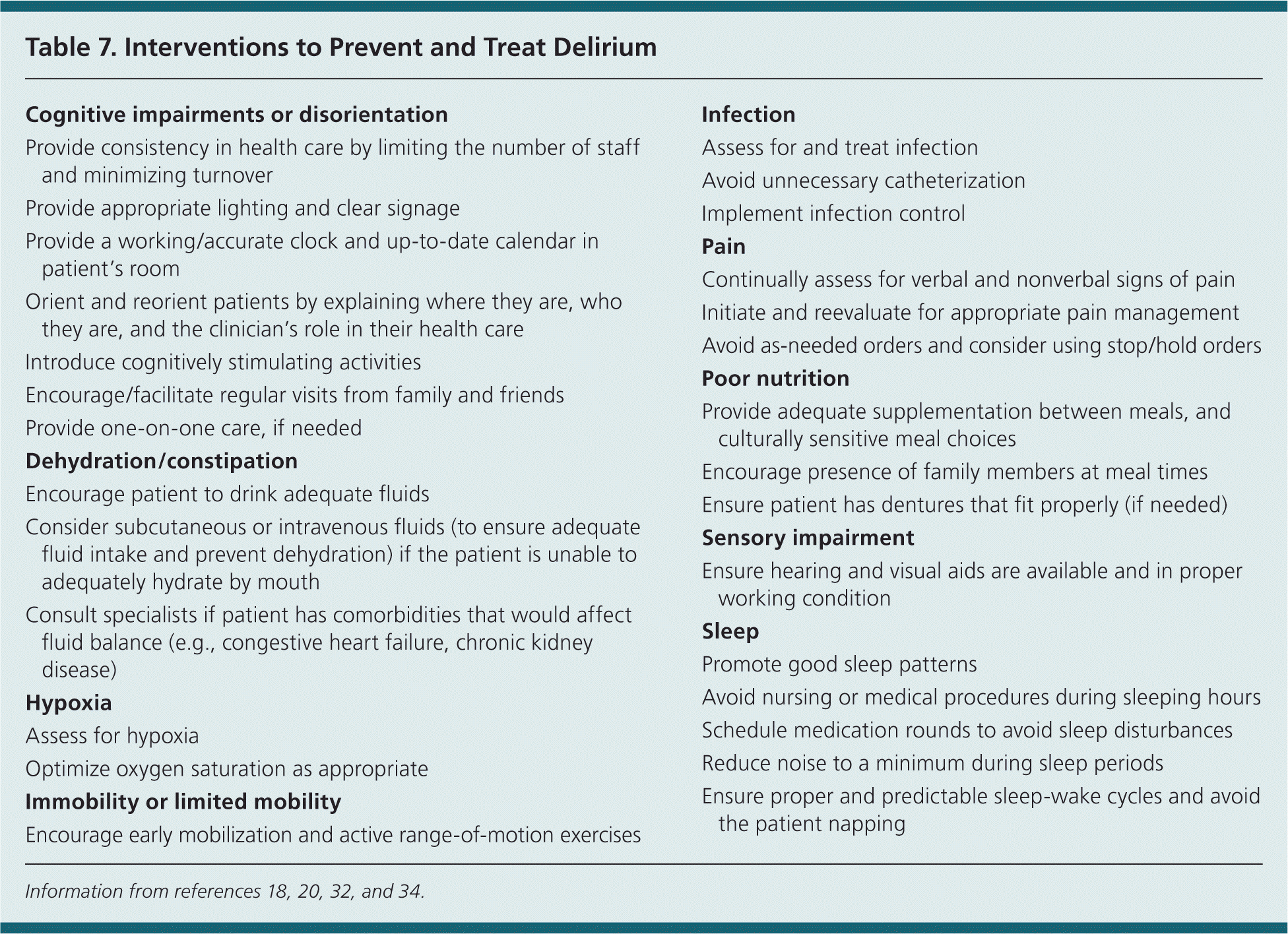
| Cognitive impairments or disorientation |
| Provide consistency in health care by limiting the number of staff and minimizing turnover |
| Provide appropriate lighting and clear signage |
| Provide a working/accurate clock and up-to-date calendar in patient's room |
| Orient and reorient patients by explaining where they are, who they are, and the clinician's role in their health care |
| Introduce cognitively stimulating activities |
| Encourage/facilitate regular visits from family and friends |
| Provide one-on-one care, if needed |
| Dehydration/constipation |
| Encourage patient to drink adequate fluids |
| Consider subcutaneous or intravenous fluids (to ensure adequate fluid intake and prevent dehydration) if the patient is unable to adequately hydrate by mouth |
| Consult specialists if patient has comorbidities that would affect fluid balance (e.g., congestive heart failure, chronic kidney disease) |
| Hypoxia |
| Assess for hypoxia |
| Optimize oxygen saturation as appropriate |
| Immobility or limited mobility |
| Encourage early mobilization and active range-of-motion exercises |
| Infection |
| Assess for and treat infection |
| Avoid unnecessary catheterization |
| Implement infection control |
| Pain |
| Continually assess for verbal and nonverbal signs of pain |
| Initiate and reevaluate for appropriate pain management |
| Avoid as-needed orders and consider using stop/hold orders |
| Poor nutrition |
| Provide adequate supplementation between meals, and culturally sensitive meal choices |
| Encourage presence of family members at meal times |
| Ensure patient has dentures that fit properly (if needed) |
| Sensory impairment |
| Ensure hearing and visual aids are available and in proper working condition |
| Sleep |
| Promote good sleep patterns |
| Avoid nursing or medical procedures during sleeping hours |
| Schedule medication rounds to avoid sleep disturbances |
| Reduce noise to a minimum during sleep periods |
| Ensure proper and predictable sleep-wake cycles and avoid the patient napping |
Pharmacologic therapy should be reserved for patients who are a threat to their own safety or the safety of others. By convention, haloperidol has been the agent of choice for treatment of delirium, despite a higher incidence of extrapyramidal adverse effects.36,37 Haloperidol has the most evidence for this indication; comparison trials of delirium treatment with haloperidol and atypical antipsychotics have been limited to small studies in which no differences in effectiveness were found.36,37 Antipsychotic medications should be used as a last resort in treating delirium and should not be used indiscriminately in persons with delirium who have not been properly evaluated.37–44 Preliminary evidence suggests that melatonin and melatonin agonists may help prevent delirium in hospitalized elderly patients.42 [ corrected] More research in the area of pharmacologic management of delirium is needed. A list of medications and their risks is provided in Table 8.36,37,43,44
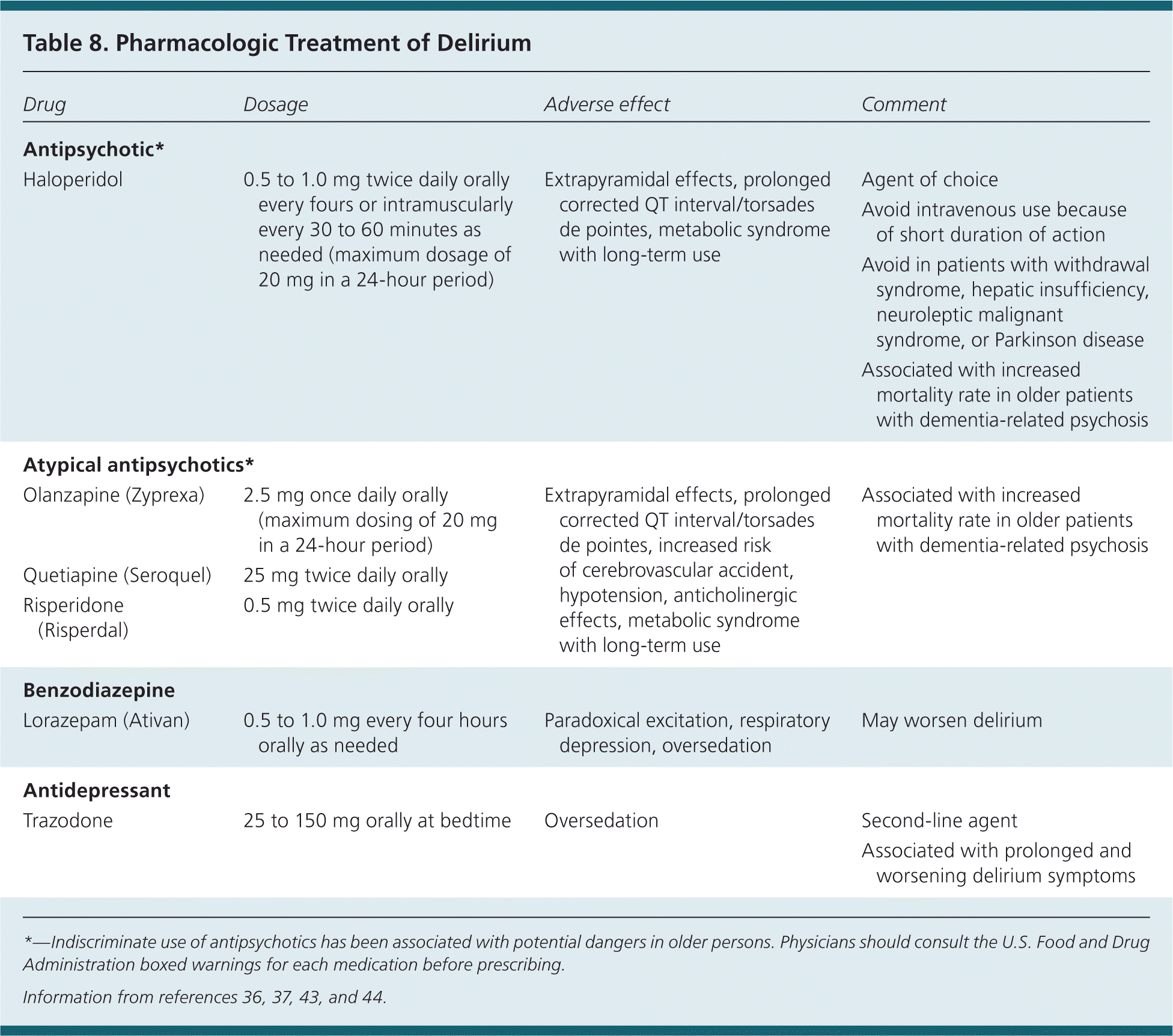
| Drug | Dosage | Adverse effect | Comment |
|---|---|---|---|
| Antipsychotic* | |||
|
|
|
|
| Atypical antipsychotics* | |||
| Olanzapine (Zyprexa) | 2.5 mg once daily orally (maximum dosing of 20 mg in a 24-hour period) | Extrapyramidal effects, prolonged corrected QT interval/torsades de pointes, increased risk of cerebrovascular accident, hypotension, anticholinergic effects, metabolic syndrome with long-term use | Associated with increased mortality rate in older patients with dementia-related psychosis |
| Quetiapine (Seroquel) | 25 mg twice daily orally | ||
| Risperidone (Risperdal) | 0.5 mg twice daily orally | ||
| Benzodiazepine | |||
| Lorazepam (Ativan) | 0.5 to 1.0 mg every four hours orally as needed | Paradoxical excitation, respiratory depression, oversedation | May worsen delirium |
| Antidepressant | |||
| Trazodone | 25 to 150 mg orally at bedtime | Oversedation |
|
COMMUNITY
A core principle of care in the home is the development of an individualized care plan that manages conditions independently. Physicians can provide a plan with parameters for the nonpharmacologic and pharmacologic management of problem behaviors. Although not developed for use by caregivers and family members, potential medical and psychiatric events requiring formal medical attention are described in Table 9.27,45
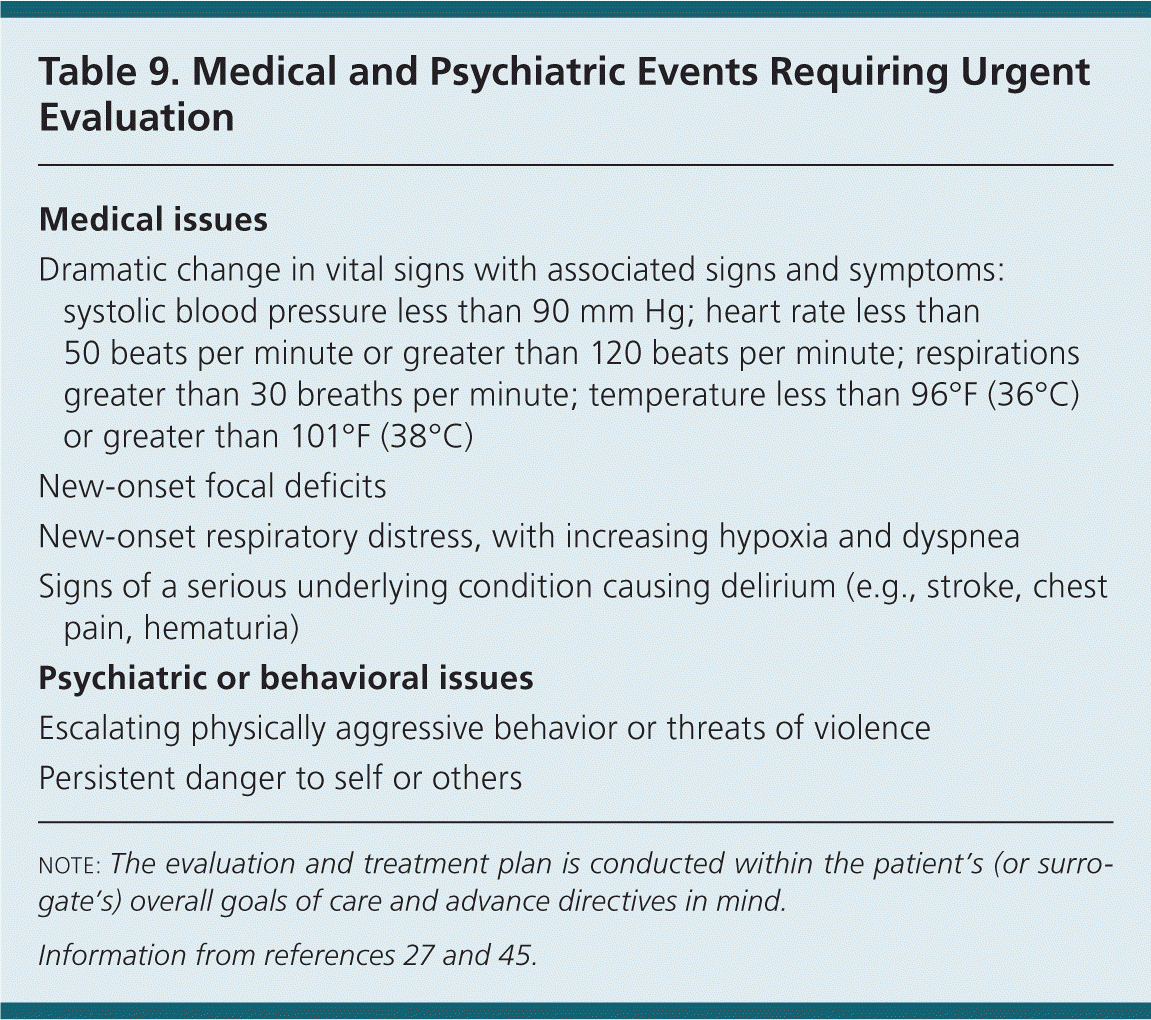
| Medical issues |
| Dramatic change in vital signs with associated signs and symptoms: systolic blood pressure less than 90 mm Hg; heart rate less than 50 beats per minute or greater than 120 beats per minute; respirations greater than 30 breaths per minute; temperature less than 96°F (36°C) or greater than 101°F (38°C) |
| New-onset focal deficits |
| New-onset respiratory distress, with increasing hypoxia and dyspnea |
| Signs of a serious underlying condition causing delirium (e.g., stroke, chest pain, hematuria) |
| Psychiatric or behavioral issues |
| Escalating physically aggressive behavior or threats of violence |
| Persistent danger to self or others |
LONG-TERM CARE FACILITY
The American Medical Directors Association has developed a clinical practice guideline for the management and treatment of delirium in long-term care facilities that attends to the medical, psychiatric, legal, cultural, environmental, and ethical issues of individual residents.45 The general principle behind the guideline embraces structured multidisciplinary assessment and treatment of delirium.
NEARING DEATH
Context-specific, nonpharmacologic interventions for delirium are still advised in this setting, but tranquilizers, such as haloperidol or atypical antipsychotics, are often required.46 Palliative sedation with agents such as benzodiazepines, propofol (Diprivan), and opioids is required for control of delirium in some patients.6,46,47
Prognosis
A meta-analysis found that delirium in hospitalized older persons was associated with increased mortality, regardless of confounders such as age, sex, and comorbidities.4 The mortality rate associated with delirium in patients in the hospital is estimated to be 14.5% to 37%.7 Prognosis hinges on the subtype and duration of delirium. Hypoactive delirium has a worse prognosis.48 A prolonged state of delirium is associated with poorer outcomes, including functional decline, dementia, and death.4,5,8,9,12 When delirium is unresolved at hospital discharge, functional and cognitive outcomes are poor.3,5
Data Sources: PubMed, Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, Essential Evidence Plus, the Institute for Clinical Systems Improvement, and the National Guideline Clearinghouse database were searched using the key terms delirium, confusion, and restraints. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: May 27, 2011, and May 26, 2014.
