
Am Fam Physician. 2014;90(3):168-175
Related editorial: Cosmetic Procedures in Family Medicine
Patient information: See related handout on cosmetic botulinum toxin injection, written by the author of this article.
Author disclosure: No relevant financial affiliations.
Botulinum toxin injection for treatment of facial wrinkles is the most frequently performed cosmetic procedure in the United States, and it is one of the most common entry procedures for clinicians seeking to incorporate aesthetic treatments into their practice. Treatment of frown lines and crow's feet, which are the cosmetic indications approved by the U.S. Food and Drug Administration, and horizontal forehead lines, offers predictable results, has few adverse effects, and is associated with high patient satisfaction. Wrinkles are formed by dermal atrophy and repetitive contraction of underlying facial musculature. Botulinum toxin is a potent neurotoxin that inhibits release of acetylcholine at the neuromuscular junction. Injection of small quantities of botulinum toxin into specific overactive muscles causes localized muscle relaxation that smooths the overlying skin and reduces wrinkles. Botulinum toxin effects take about two weeks to fully develop and last three to four months. Dynamic wrinkles, seen during muscle contraction, yield more dramatic results than static wrinkles, which are visible at rest. Botulinum toxin injection is contraindicated in persons with keloidal scarring, neuromuscular disorders (e.g., myasthenia gravis), allergies to constituents of botulinum toxin products, and body dysmorphic disorder. Minor bruising can occur with botulinum toxin injection. Temporary blepharoptosis and eyebrow ptosis are rare complications that are technique-dependent; incidence declines as injector skill improves.
Botulinum toxin injection for treatment of facial wrinkles is the most frequently performed cosmetic procedure in the United States,1 and it is one of the most common entry procedures for clinicians seeking to incorporate aesthetic treatments into their practice.2 Botulinum toxin injection, particularly in the upper one-third of the face, offers predictable results,3 has few adverse effects,4 and is associated with high patient satisfaction.5 This article reviews relevant facial anatomy, patient selection, complications, and injection technique for cosmetic botulinum toxin treatment with a focus on frown lines. It is, however, not intended as a replacement for a formal instructional course.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Botulinum toxin serotype A is safe and effective for reduction of frown lines. | A | 9, 12, 13, 20, 21 |
| Botulinum toxin serotype A is safe and effective for reduction of crow's feet. | A | 12, 21, 24, 25 |
| In preparation for botulinum toxin treatment, patients should be advised to discontinue aspirin and any medication or dietary supplements associated with bruising for two weeks before treatment. | C | 17, 23 |
Mechanism of Action
Wrinkles are formed by dermal atrophy and repetitive contraction of underlying facial musculature. Injection of small quantities of botulinum toxin into specific overactive muscles causes localized muscle relaxation that smooths the overlying skin and reduces wrinkles.6
Botulinum toxin is a potent neurotoxin protein derived from the Clostridium botulinum bacterium. It exerts its effect at the neuromuscular junction by inhibiting the release of acetylcholine, which causes temporary chemical denervation. At the cellular level, botulinum toxin functions by cleaving a docking protein (synaptosomal-associated protein of 25 kDA [SNAP-25]) on the internal surface of neuronal membranes, thereby inhibiting vesicle fusion and release of acetylcholine.7 Botulinum toxin effects in the targeted muscles diminish over time as SNAP-25 regenerates, and neuromuscular signaling and muscle contractility are restored.
Indications
Botulinum toxin has been used for more than 20 years to treat a variety of conditions including blepharospasm, strabismus, cervical dystonia, migraines, hyperhidrosis, and muscle spasticity. Botulinum toxin was first approved by the U.S. Food and Drug Administration (FDA) for cosmetic use in 2002 as Botox to treat glabellar complex muscles that form frown lines and in 2013 to treat lateral orbicularis oculi muscles that form crow's feet 8; it is used off-label for all other cosmetic facial indications. It has become the treatment of choice for wrinkles occurring in the upper one-third of the face (i.e., frown lines, horizontal forehead lines, and crow's feet). It is also used in the lower two-thirds of the face, but this is more technically challenging and is an advanced application.9–14
Anatomy
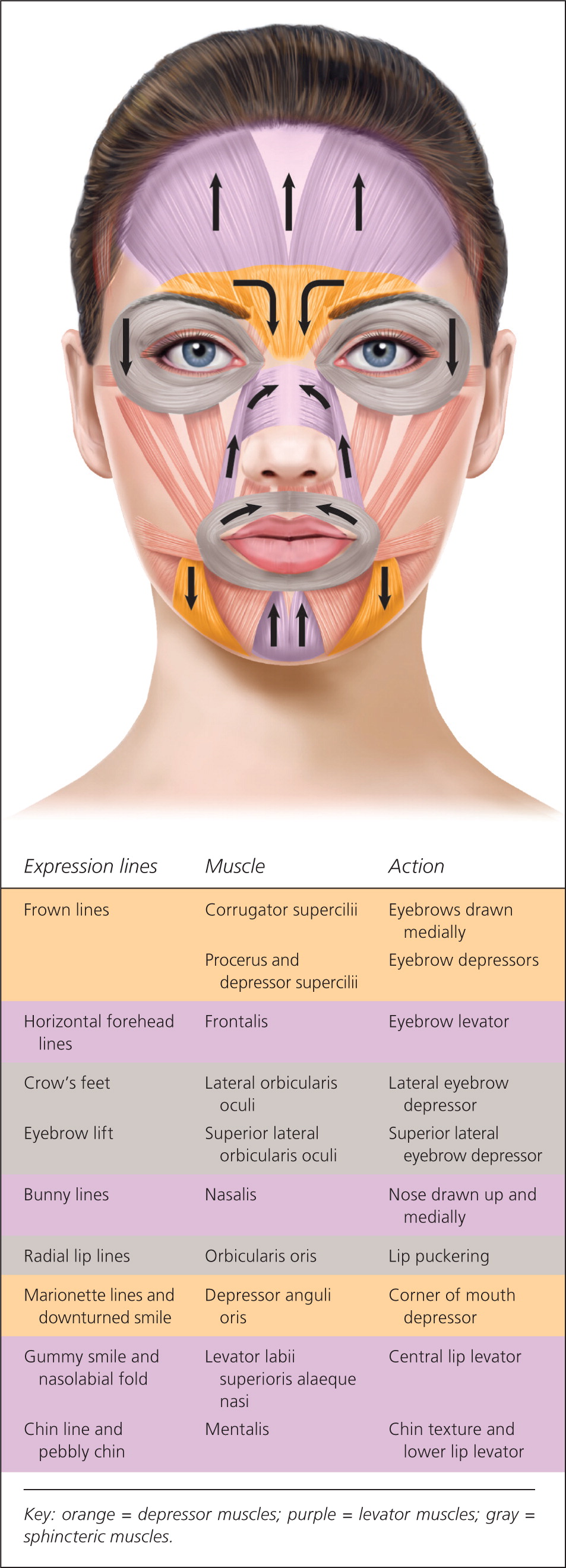
The muscles of facial expression are unique in that they have soft tissue attachments to skin through the superficial muscular aponeurotic system, unlike most muscles, which have bony attachments (Figure 115 ). When facial muscles contract, the overlying skin also moves, forming dynamic wrinkles perpendicular to the direction of muscle contraction (Figure 215 ).
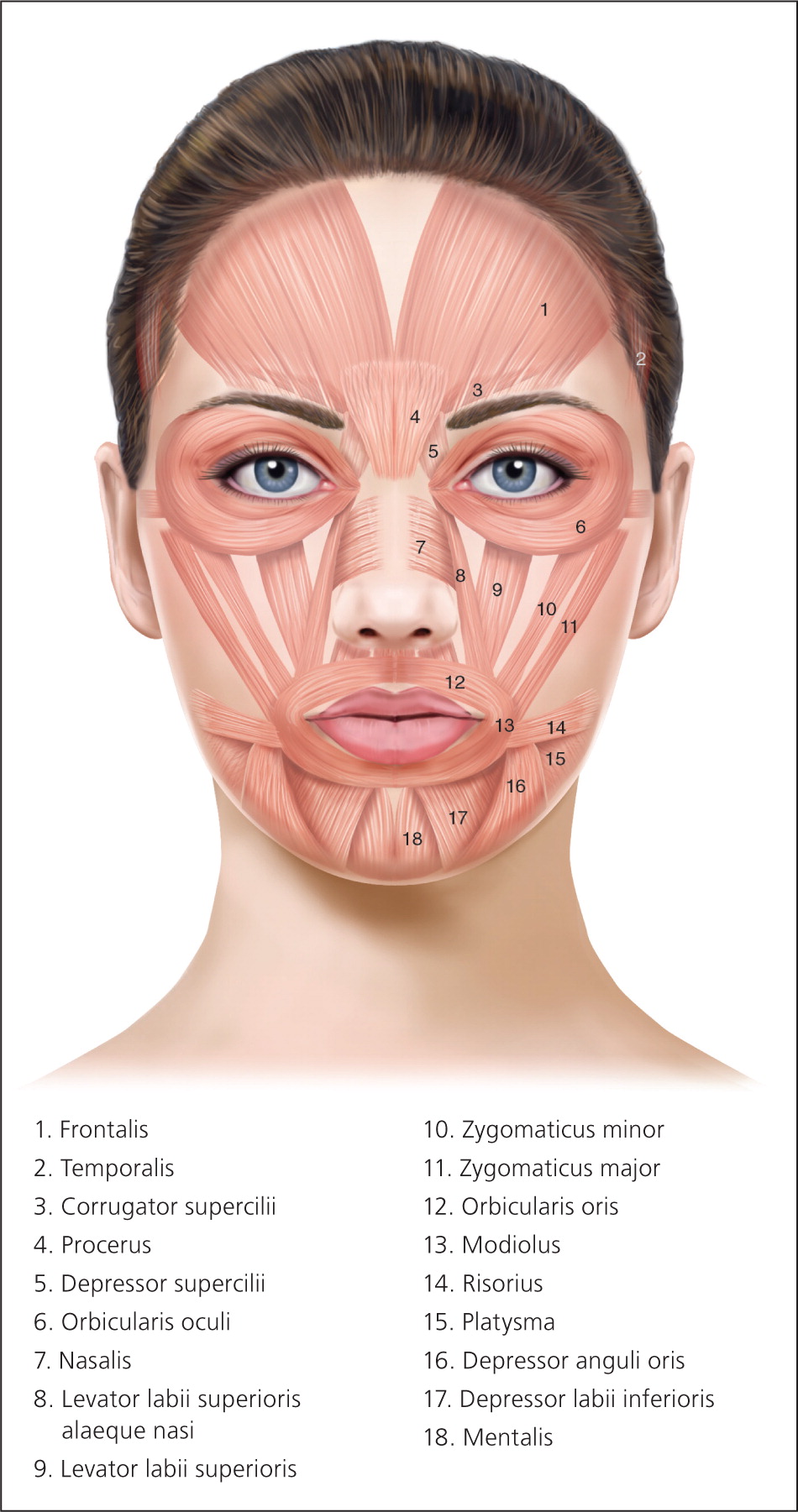
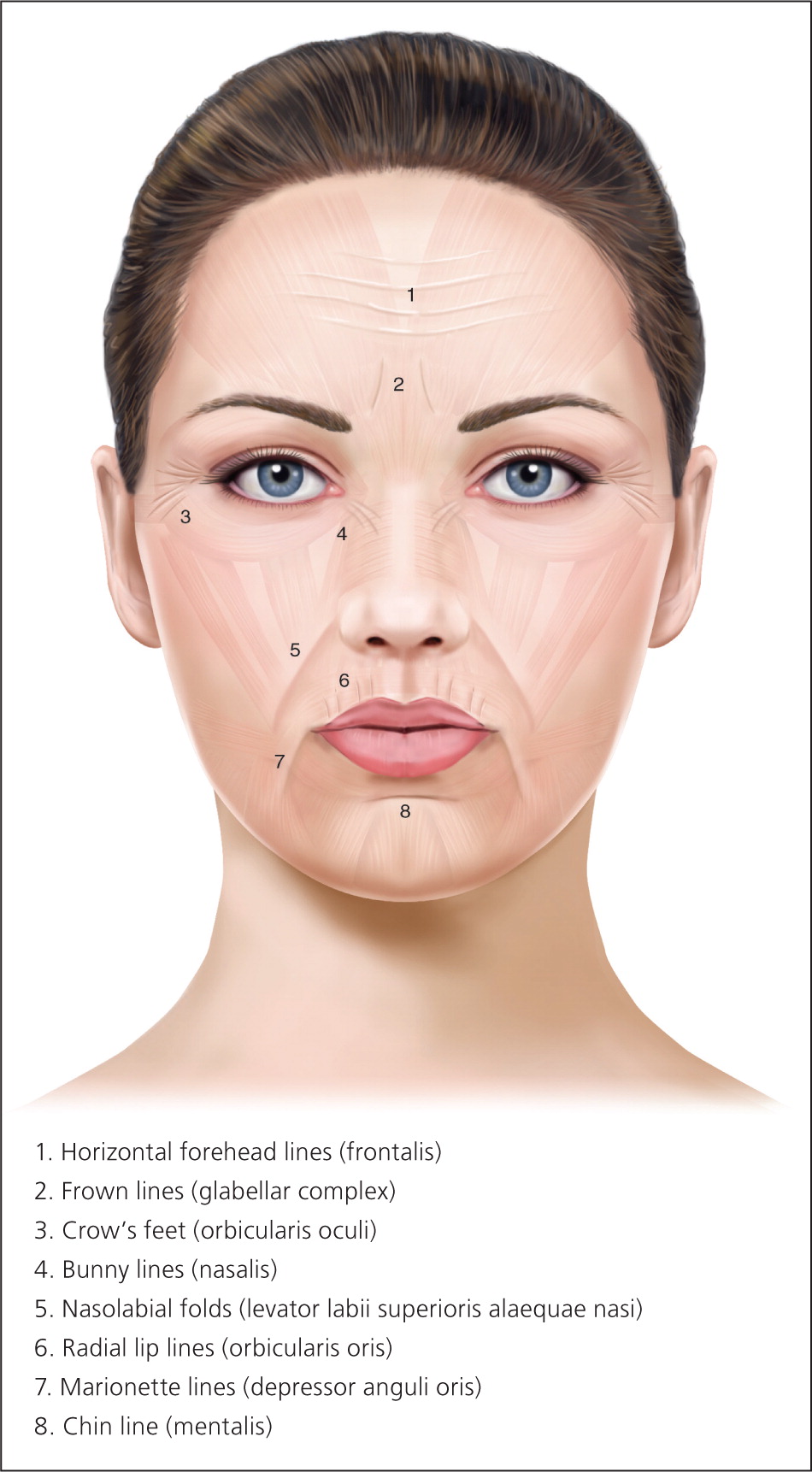
Glabellar wrinkles, or frown lines, are vertical lines occurring between the medial aspects of the eyebrows. The muscles contributing to formation of frown lines are the glabellar complex depressor muscles, which include the corrugator supercilii, procerus, and depressor supercilii. Contraction of these muscles pulls the brows medially and inferiorly (Figure 315 ).
Patient Selection
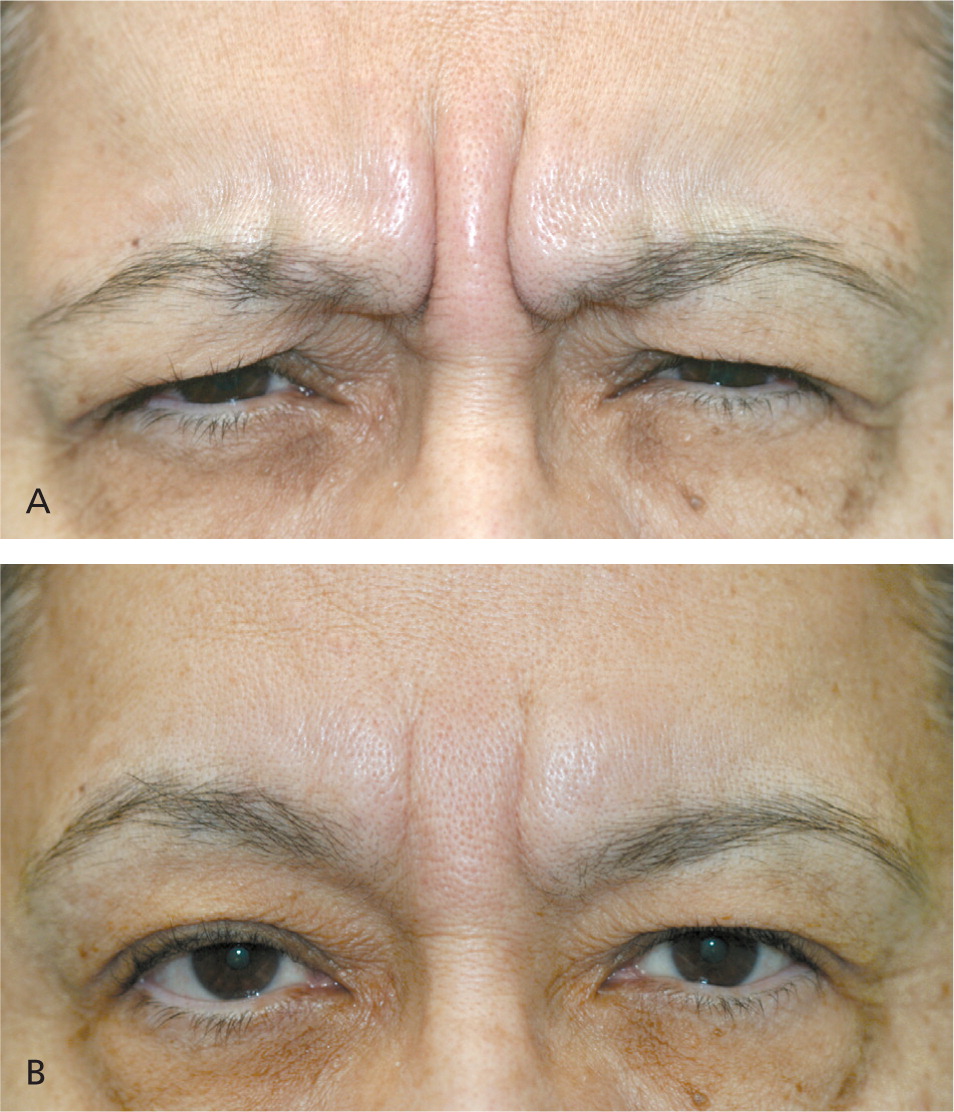
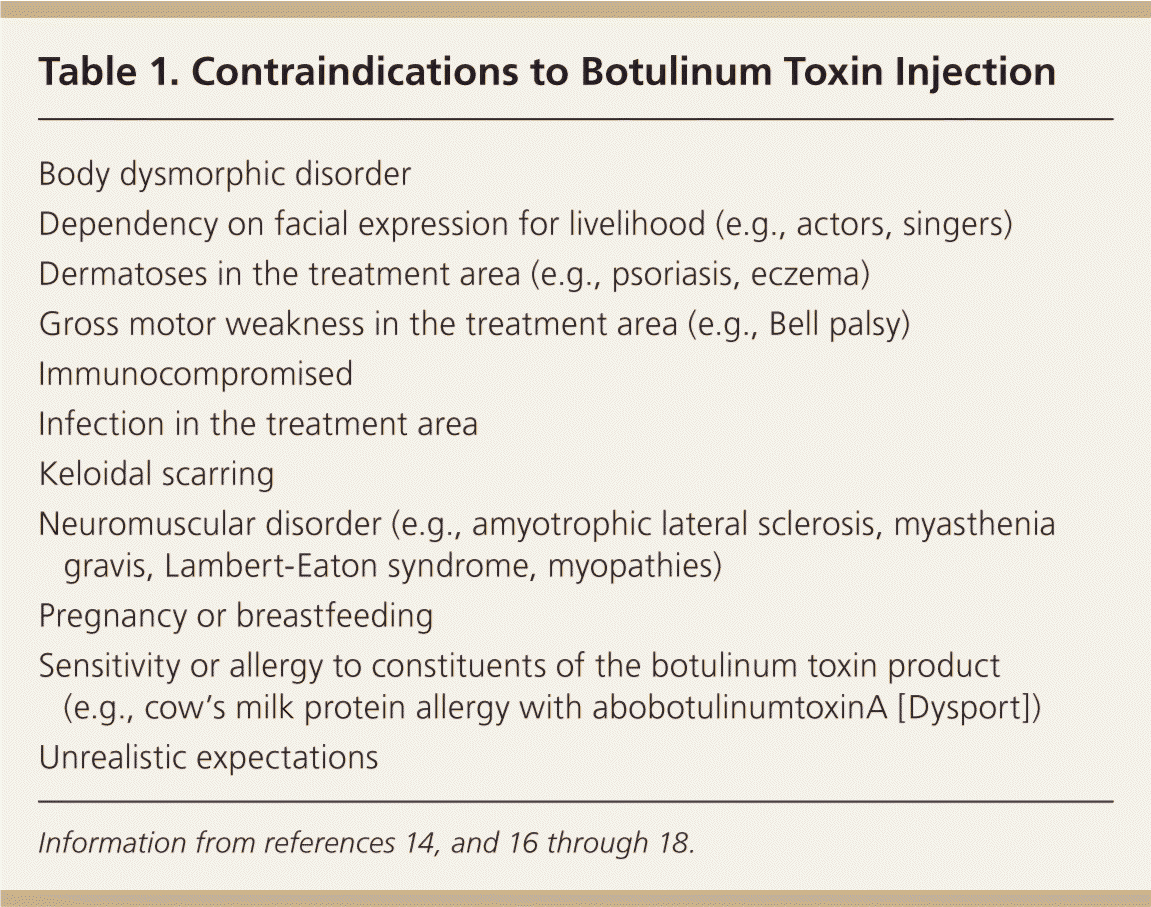
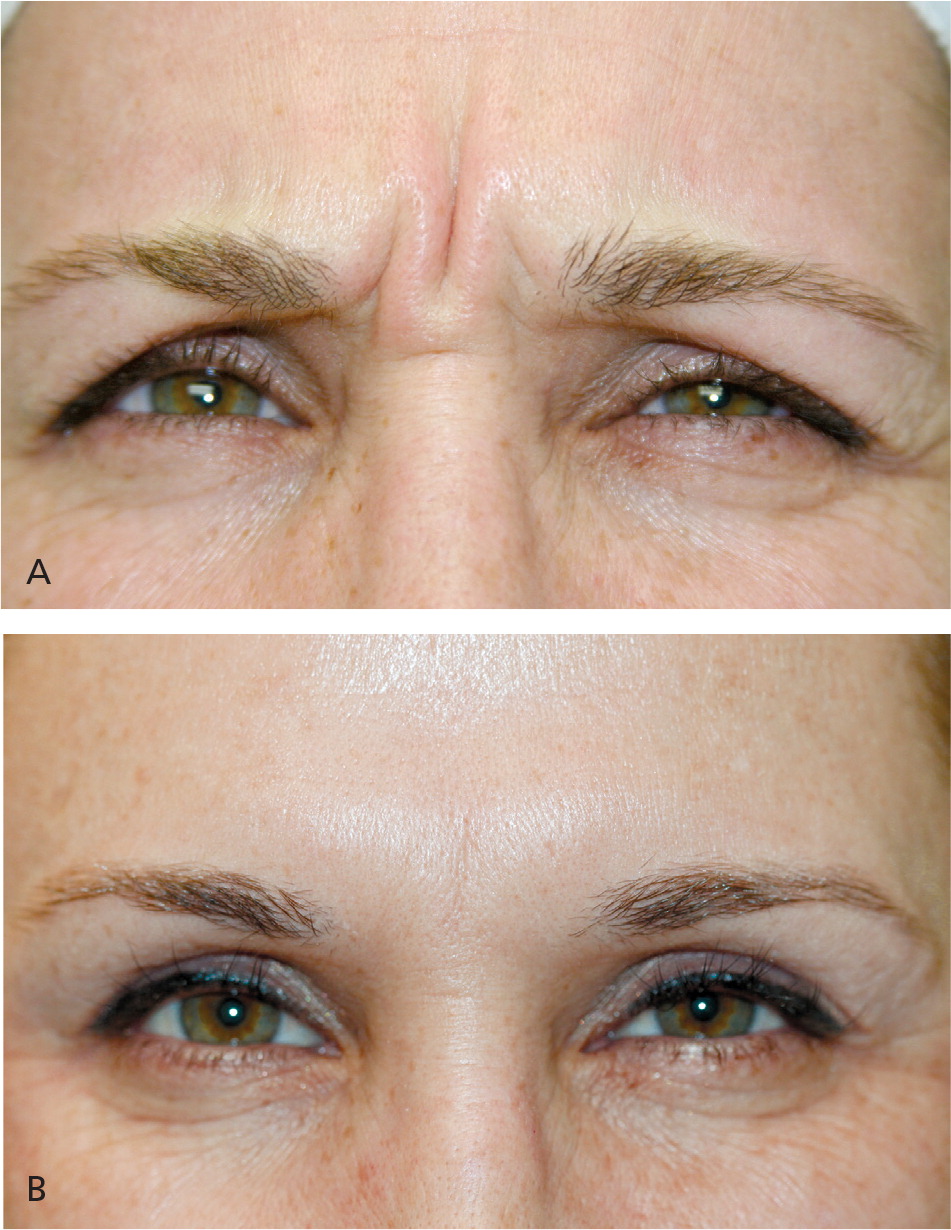
Patients with dynamic wrinkles demonstrate the most dramatic improvements from botulinum toxin injection and are ideal candidates for treatment (Figure 415 ). Patients with static wrinkles that are visible at rest are also candidates (Figure 515 ), but results are slower and patients may require two or three consecutive botulinum toxin treatments for significant improvements.15 Deep static lines may not fully respond to botulinum toxin injection alone and may require combination treatment with dermal fillers or other cosmetic procedures to achieve optimal results. Setting realistic expectations at the time of consultation is important for patient satisfaction and success with botulinum toxin treatments. Contraindications to botulinum toxin injection include keloidal scarring, neuromuscular disorders (e.g., myasthenia gravis), allergy to constituents of botulinum toxin product, unrealistic expectations, and body dysmorphic disorder (Table 1).14,16–18
| Body dysmorphic disorder |
| Dependency on facial expression for livelihood (e.g., actors, singers) |
| Dermatoses in the treatment area (e.g., psoriasis, eczema) |
| Gross motor weakness in the treatment area (e.g., Bell palsy) |
| Immunocompromised |
| Infection in the treatment area |
| Keloidal scarring |
| Neuromuscular disorder (e.g., amyotrophic lateral sclerosis, myasthenia gravis, Lambert-Eaton syndrome, myopathies) |
| Pregnancy or breastfeeding |
| Sensitivity or allergy to constituents of the botulinum toxin product(e.g., cow's milk protein allergy with abobotulinumtoxinA [Dysport]) |
| Unrealistic expectations |
Botulinum Toxin Products
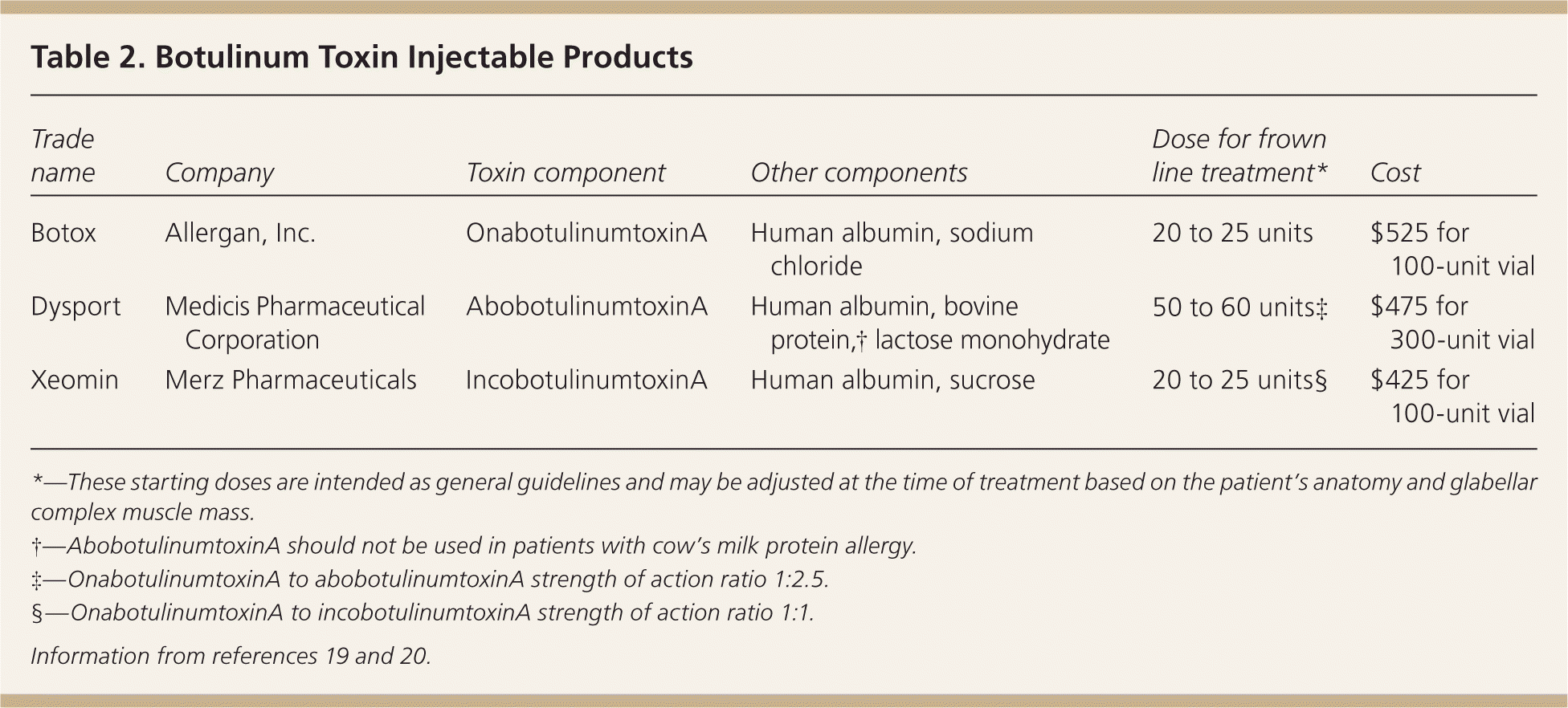
The C. botulinum bacterium produces eight serotypes of botulinum toxin protein (A, B, Cα, Cβ, D, E, F, and G). Botulinum toxin serotype A is the most potent and is used for cosmetic treatments. Botulinum toxin serotype B is used for medical conditions such as dystonia. There are currently three botulinum toxin serotype A products approved by the FDA for cosmetic use to treat glabellar complex muscles that form frown lines: onabotulinumtoxinA (Botox), abobotulinumtoxinA (Dysport), and incobotulinumtoxinA (Xeomin), which are summarized in Table 2.19,20 All three serotype A products have a 150 kDa core botulinum toxin protein and differ in whether they have complexing proteins surrounding the core neurotoxin. OnabotulinumtoxinA and abobotulinumtoxinA have hemagglutinin complexing proteins, whereas incobotulinumtoxinA is free from complexing proteins.19,21 Lack of complexing proteins may reduce antigenicity and hence antibody formation against botulinum toxin, but the clinical significance of complexing proteins has yet to be determined. Botulinum toxin products are not interchangeable because they vary in their formulation, dosing, and clinical response.
| Trade name | Company | Toxin component | Other components | Dose for frown line treatment* | Cost |
|---|---|---|---|---|---|
| Botox | Allergan, Inc. | OnabotulinumtoxinA | Human albumin, sodium chloride | 20 to 25 units | $525 for 100-unit vial |
| Dysport | Medicis Pharmaceutical Corporation | AbobotulinumtoxinA | Human albumin, bovine protein,† lactose monohydrate | 50 to 60 units‡ | $475 for 300-unit vial |
| Xeomin | Merz Pharmaceuticals | IncobotulinumtoxinA | Human albumin, sucrose | 20 to 25 units§ | $425 for 100-unit vial |
Aesthetic Consultation
Facial areas of concern are assessed by the physician and patient simultaneously using a handheld mirror during the consultation. Asymmetries such as uneven eyebrow height and eye aperture are identified. Areas are prioritized and treatment options discussed, including anticipated results and possible complications. Botulinum toxin is the only treatment for dynamic wrinkles currently approved by the FDA. Treatment options for static wrinkles include resurfacing procedures (e.g., chemical peels, microdermabrasion, lasers), topical products (e.g., retinoids), and surgical procedures. Photographic documentation is recommended with any aesthetic procedure. Dynamic and static photographs of treatment areas are typically taken before treatment and two weeks after treatment, once clinical effects are evident.22
Procedure Preparation
In preparation for botulinum toxin treatment, or any injectable procedure, bruising can be minimized by advising patients to discontinue aspirin and any medication or dietary supplement that has anticoagulant effects two weeks before treatment.17,23 Anesthesia is not typically necessary for botulinum toxin treatments.
Botulinum Toxin Injection
Botulinum toxin is supplied as a powder and is reconstituted at the time of treatment into a solution using sterile normal saline. Dilution volumes range from 1 to 4 mL per 100-unit vial. The botulinum toxin dose injected into glabellar complex muscles for the treatment of frown lines is based on the specific botulinum toxin product used and mass of the target muscles. Table 2 lists starting doses used for treatment of frown lines with the three FDA-approved botulinum toxin products.19,20
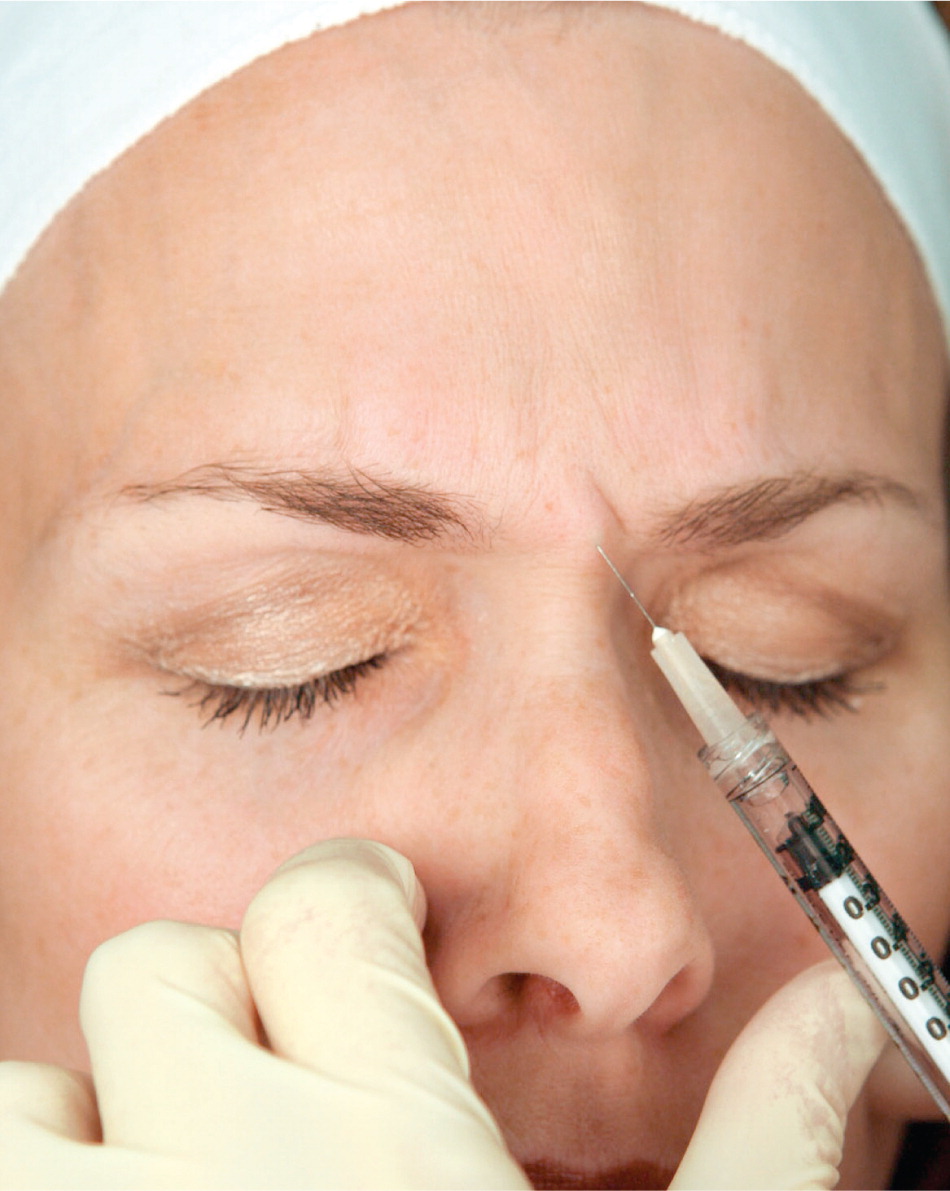
The targeted glabellar complex muscles can be identified by having the patient actively frown, and injections are placed into the contracted muscles (Figure 615 ). Small volumes of botulinum toxin solution are injected, typically 1 mL or less, using a 30-gauge, 1-inch needle. There are five injection sites, one injection in the procerus muscle and two in each of the corrugator supercilii muscles.15,20,24 Botulinum toxin is commonly used to treat other lines in the upper one-third of the face, such as horizontal forehead lines with injection in the frontalis muscle, and crow's feet with injection in the lateral orbicularis oculi muscles.10,11,25 Localized burning or stinging sensation during injection is commonly reported and resolves within a few minutes.15,17
Aftercare
Patients are advised to avoid lying supine following treatment for four hours. They are also advised to avoid massaging or applying heat to the treatment area, and to avoid activities that cause flushing (such as exercising heavily, consuming alcohol, and hot tub use) on the day of treatment.15,26 These aftercare practices are used to reduce potential spread of the toxin, however; they are not supported by randomized controlled trials.
Results and Follow-Up
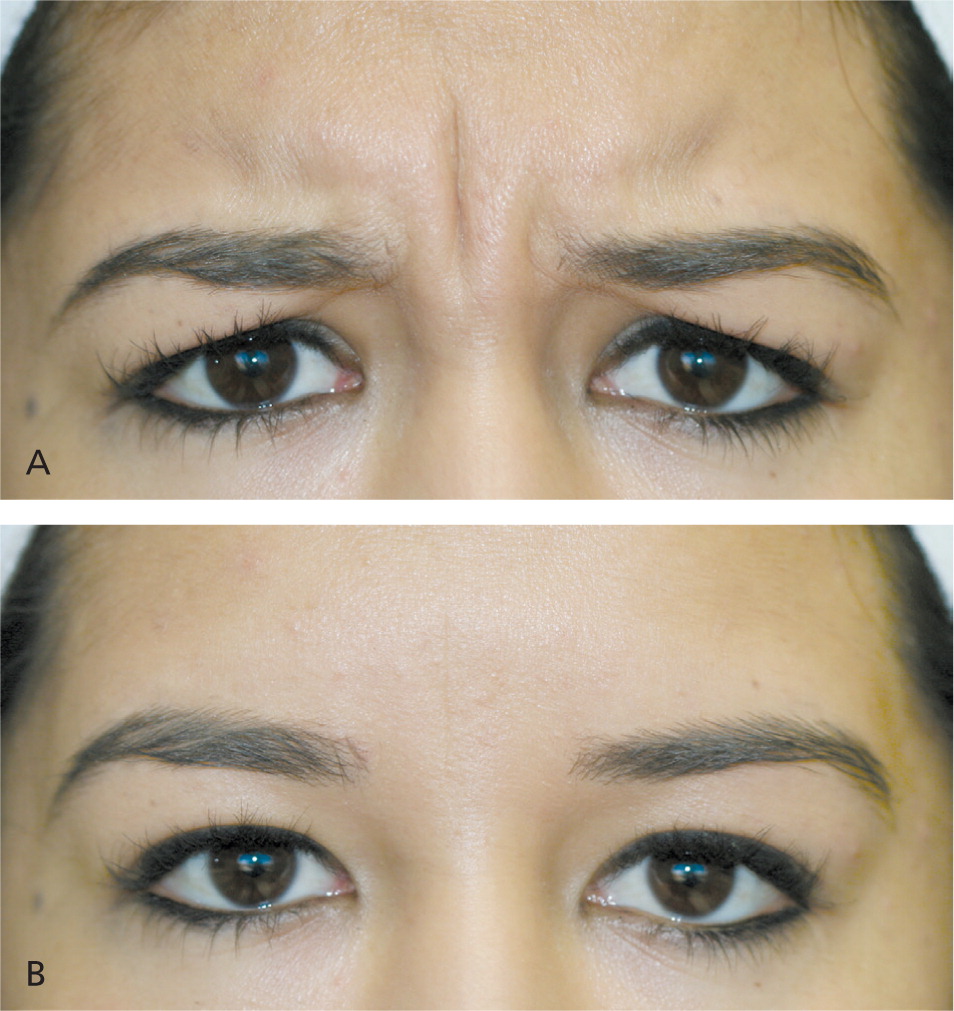
Partial reduction in function of the targeted glabellar complex muscles is seen by the third day after botulinum toxin injection, with maximal reduction visible two weeks after injection.27 Figure 7 shows reduction of dynamic frown lines one month after treatment of the glabellar complex muscles with 20 units of onabotulinumtoxinA.15 Return of muscle function is gradual, typically three to four months after treatment. Subsequent treatment is advised when muscle contraction is visible in the treatment area before facial lines return to their pretreatment appearance.28 After multiple treatments, botulinum toxin effects may be prolonged and, for some patients, treatment intervals can be extended beyond three to four months.29
Complications

| Injection reactions |
| Anxiety or vasovagal episode |
| Ecchymosis |
| Erythema, edema, and tenderness |
| Headache |
| Infection |
| Pain |
| Paresthesia or dysesthesia |
| Undesired botulinum toxin effects |
| Allergic reaction |
| Antibodies against botulinum toxin |
| Blepharoptosis |
| Distant spread from the injection site |
| Eyebrow ptosis |
| Facial asymmetry |
| Medication interactions |
| Undesired eyebrow shape or unsatisfactory result |
INJECTION REACTIONS
Botulinum toxin injections are performed using small-gauge needles to minimize discomfort and bruising. Mild erythema, edema, and tenderness at injection sites are expected and resolve within a day. Bruising is common and ranges from pinpoint needle insertion marks to quarter-sized ecchymoses that can take up to two weeks to resolve. Application of ice and pressure to a bruise can minimize enlargement.15 Headaches can occur with facial injections; most are mild and spontaneously resolve a few days after treatment.33 There are reports of idiosyncratic severe headaches lasting two to four weeks.34 Nonsteroidal anti-inflammatory drugs or opioids may be used to manage headaches based on severity. Infection is rare, but can occur with any procedure that breaches the skin barrier. Paresthesia or dysesthesia in the treatment area is rare, and may be caused by nerve trauma. Anxiety with injection procedures is common. Vasovagal episodes associated with severe anxiety can occur and it is advisable to have appropriate emergency protocols and medications available in the office when performing injection procedures.35
UNDESIRED EFFECTS
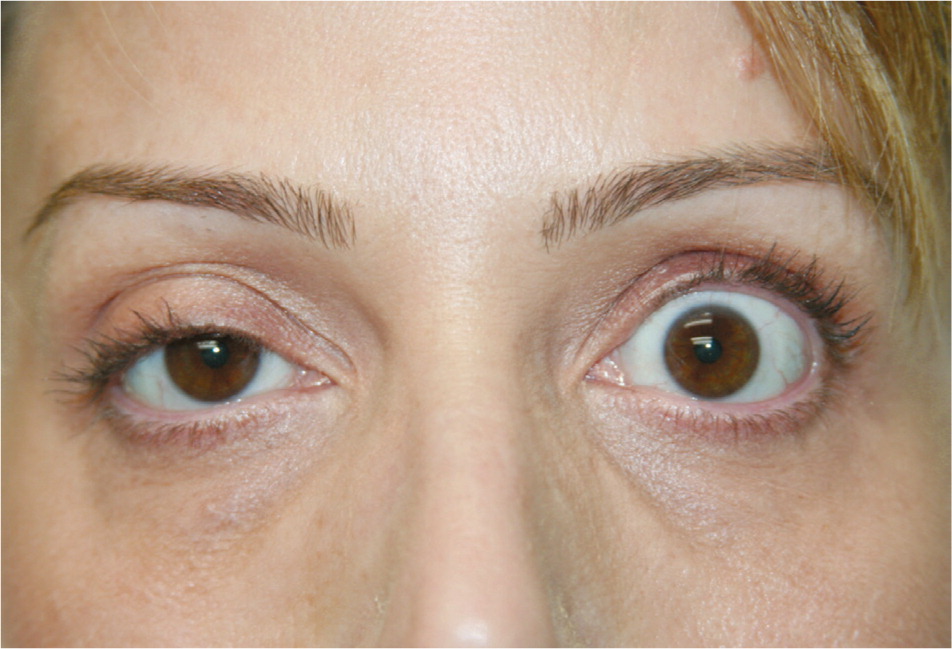
Complications related to botulinum toxin effects occur less frequently than injection reactions, and are primarily caused by temporary denervation of adjacent muscles outside of the intended treatment area. These complications are technique-dependent; incidence declines as injector skill improves.30,31 Temporary blepharoptosis (upper eyelid droop) is uncommon (1% to 5%) but is distressing for patients.9 It is almost always unilateral, seen as a 2- to 3-mm lowering of the affected eyelid that is most marked at the end of the day with muscle fatigue (Figure 815 ). Blepharoptosis is caused by deep migration of botulinum toxin through the orbital septum fascia to the levator palpebrae superioris, an upper eyelid levator muscle. Incidence of blepharoptosis is reduced by placing botulinum toxin injections at least 1 cm above the supraorbital ridge at the midpupillary line when treating the corrugator muscles.14,17 Blepharoptosis may be treated using ophthalmic solutions that have alpha-adrenergic effects, such as over-the-counter naphazoline 0.025%/pheniramine 0.3% or prescription apraclonidine 0.5% (Iopidine). Both medications cause contraction of Müller muscle, an adrenergic levator muscle of the upper eyelid, resulting in elevation of the upper eyelid. Apraclonidine is reserved for refractory cases and should be used with caution because it can exacerbate or unmask underlying glaucoma.32 Eyebrow ptosis and undesired eyebrow shape are usually related to unintended botulinum toxin effects in the frontalis muscle. Some of these complications can be corrected with botulinum toxin injection in muscles that antagonize the affected muscles; however, complications caused by involvement of adjacent muscles are temporary and will spontaneously resolve as botulinum toxin effects diminish. Facial asymmetry can result from uneven dosing of botulinum toxin. Consistent technique and careful attention to injection volumes at the time of treatment can reduce the incidence of asymmetries.
Other rare complications associated with botulinum toxin injections include formation of antibodies (less than 1%), which can render treatments ineffective.36 Medications that inhibit neuromuscular signaling such as aminoglycosides, quinidine, anticholinergics, and muscle relaxants, may potentiate botulinum toxin effects.37 AbobotulinumtoxinA is contraindicated in patients with cow's milk protein allergy because bovine protein is a constituent.38,39 Although extremely rare, immediate hypersensitivity and allergic reactions may occur, with signs of urticaria, edema, and possibly anaphylaxis. Using standard emergency protocols and medications such as epinephrine and methylprednisolone (Solu-Medrol) is advised when indicated, rather than diphenhydramine (Benadryl) because of its anticholinergic effects.40 Case reports of adverse effects due to distant spread from the injection site have been reported with large doses of botulinum toxin (e.g., 300 units in the calves), which include generalized muscle weakness, ptosis, dysphagia, dysarthria, urinary incontinence, respiratory difficulties, and death from respiratory compromise.41 Distant spread has not been reported with cosmetic use of botulinum toxin for frown lines or other facial indications. There are case reports of botulism with injected botulinum toxin; however, these instances involved research-grade botulinum toxin that was not approved or intended for human use.42,43
Financial Considerations and Coding
The current procedural terminology (CPT) designation for botulinum toxin injection of the face is chemodenervation of muscles innervated by the facial nerve (CPT code: 64612). Cosmetic botulinum toxin treatments are not covered by insurance. Fees for injections range from $250 to $550 per treatment area and are based on the number of botulinum toxin units used or on the area treated (i.e., frown lines, crow's feet, or horizontal forehead lines).
Data Sources: A PubMed, Cochrane database, POEMs, and Essential Evidence search was completed in Clinical Queries using the key terms botulinum toxin, safety, efficacy, cosmetic, and frown lines. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the FDA website, aesthetic procedure textbooks, and UpToDate. Search dates: February 2, 2013, and April 24, 2014.
