A more recent article on care of premature infants is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2014;90(4):244-251
Related letter: Vitamin D Supplementation in Premature Infants
Author disclosure: No relevant financial affiliations.
Preterm births (deliveries before 37 weeks' gestation) comprise 12% of all U.S. births and are responsible for one-third of all infant deaths. Neonatal medical advances have increased survival, and primary care physicians often care for infants who were in the neonatal intensive care unit after birth. Functions of the primary care physician include coordination of medical and social services, nutritional surveillance, and managing conditions associated with prematurity. Parental guidance and encouragement are often necessary to ensure appropriate feeding and infant weight gain. Enriched formula and nutrient fortifiers are used for infants with extrauterine growth restriction. Iron supplementation is recommended for breastfed infants, and iron-fortified formula for formula-fed infants. Screening for iron deficiency anemia in preterm infants should occur at four months of age and at nine to 12 months of age. Gastroesophageal reflux is best treated with nonpharmacologic options because medications provide no long-term benefits. Neurodevelopmental delay occurs in up to 50% of preterm infants. Developmental screening should be performed at every well-child visit. If developmental delay is suspected, more formalized testing may be required with appropriate referral. To prevent complications from respiratory syncytial virus infection, palivizumab is recommended in the first year of life during the respiratory syncytial virus season for all infants born before 29 weeks' gestation and for infants born between 29 and 32 weeks' gestation who have chronic lung disease. Most preterm infants have minimal long-term sequelae.
Preterm birth (delivery before 37 weeks' gestation) comprises 12% of all deliveries and accounts for one-third of all infant deaths.1,2 Because advances in neonatal medical care have increased the survival of preterm infants, primary care physicians are taking a more active role in the care of these infants after they are released from the neonatal intensive care unit (e.g., coordinating medical and social services, nutritional surveillance, managing conditions associated with prematurity, and parental guidance and encouragement). Common terminology used in the care of premature infants is listed in Table 1,3 and a summary schedule for well visits is provided in Table 2.4,5 This article reviews common questions encountered in managing care of premature infants.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Nutrient fortification of breast milk or enriched formula should be considered in premature infants who are less than the 10th percentile in weight for corrected age. | C | 20, 25, 29, 30, 36 |
| Standardized screening should be used in premature infants at nine, 18, and 24 to 30 months of age to detect neurodevelopmental delay. | C | 43–45, 49 |
| Breastfed preterm infants should receive supplemental iron from one to 12 months of age or until complementary foods provide a sufficient amount of iron. | C | 53, 54 |
| Immunoprophylaxis with palivizumab (Synagis) is recommended in the first year of life during the respiratory syncytial virus season for all infants born before 29 weeks' gestation to decrease the risk of hospitalization. | B | 55, 56, 58 |

| Term | Definition | ||
|---|---|---|---|
| Prematurity (based on gestational age) | |||
| Early term | 37 to < 39 weeks | ||
| Late preterm | 34 to < 37 weeks | ||
| Very premature | 32 weeks or less | ||
| Extremely premature | 25 weeks or less | ||
| Border of viability | 22 to < 25 weeks | ||
| Birth weight | |||
| Low birth weight | Less than 2,500 g (5 lb, 8 oz) | ||
| Very low birth weight | Less than 1,500 g (3 lb, 5 oz) | ||
| Extremely low birth weight | Less than 1,000 g (2 lb, 3 oz) | ||
| Age | |||
| Gestational | Elapsed time between last menstrual period and date of delivery | ||
| Chronologic (postnatal) | Elapsed time since birth | ||
| Postmenstrual | Gestational age plus chronologic age (e.g., an infant with a gestational age of 28 weeks and a chronologic age of 8 weeks would have a postmenstrual age of 36 weeks) | ||
| Corrected | Chronologic age minus number of weeks premature (e.g., a 12-month-old born at 28 weeks' gestation has a corrected age of 9 months) | ||
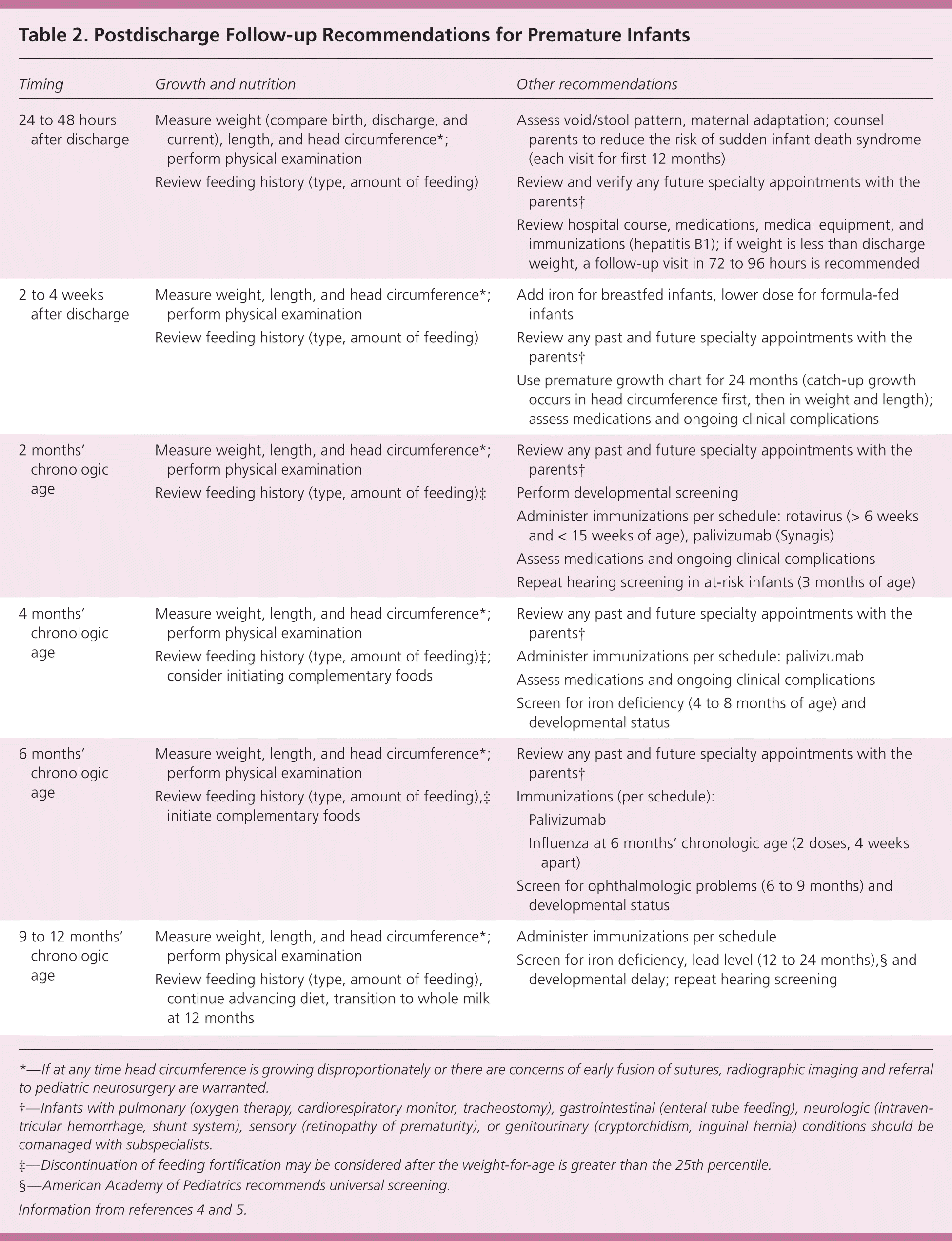
| Timing | Growth and nutrition | Other recommendations | |
|---|---|---|---|
| 24 to 48 hours after discharge | Measure weight (compare birth, discharge, and current), length, and head circumference*; perform physical examination | Assess void/stool pattern, maternal adaptation; counsel parents to reduce the risk of sudden infant death syndrome (each visit for first 12 months) | |
| Review feeding history (type, amount of feeding) | Review and verify any future specialty appointments with the parents† | ||
| Review hospital course, medications, medical equipment, and immunizations (hepatitis B1); if weight is less than discharge weight, a follow-up visit in 72 to 96 hours is recommended | |||
| 2 to 4 weeks after discharge | Measure weight, length, and head circumference*; perform physical examination | Add iron for breastfed infants, lower dose for formula-fed infants | |
| Review feeding history (type, amount of feeding) | Review any past and future specialty appointments with the parents† | ||
| Use premature growth chart for 24 months (catch-up growth occurs in head circumference first, then in weight and length); assess medications and ongoing clinical complications | |||
| 2 months' chronologic age | Measure weight, length, and head circumference*; perform physical examination | Review any past and future specialty appointments with the parents† | |
| Review feeding history (type, amount of feeding)‡ | Perform developmental screening | ||
| Administer immunizations per schedule: rotavirus (> 6 weeks and < 15 weeks of age), palivizumab (Synagis) | |||
| Assess medications and ongoing clinical complications | |||
| Repeat hearing screening in at-risk infants (3 months of age) | |||
| 4 months' chronologic age | Measure weight, length, and head circumference*; perform physical examination | Review any past and future specialty appointments with the parents† | |
| Review feeding history (type, amount of feeding)‡; consider initiating complementary foods | Administer immunizations per schedule: palivizumab | ||
| Assess medications and ongoing clinical complications | |||
| Screen for iron deficiency (4 to 8 months of age) and developmental status | |||
| 6 months' chronologic age | Measure weight, length, and head circumference*; perform physical examination | Review any past and future specialty appointments with the parents† | |
| Review feeding history (type, amount of feeding),‡ initiate complementary foods | Immunizations (per schedule): | ||
| Palivizumab | |||
| Influenza at 6 months' chronologic age (2 doses, 4 weeks apart) | |||
| Screen for ophthalmologic problems (6 to 9 months) and developmental status | |||
| 9 to 12 months' chronologic age | Measure weight, length, and head circumference*; perform physical examination | Administer immunizations per schedule | |
| Review feeding history (type, amount of feeding), continue advancing diet, transition to whole milk at 12 months | Screen for iron deficiency, lead level (12 to 24 months),§ and developmental delay; repeat hearing screening | ||
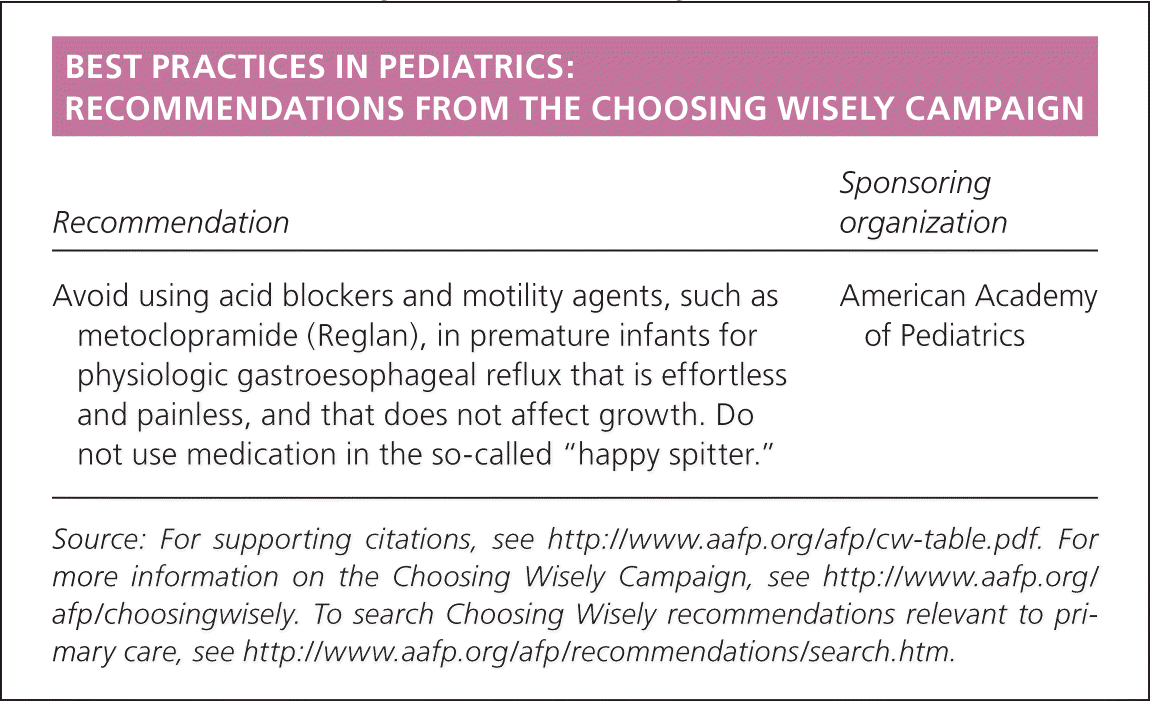
| Recommendation | Sponsoring organization |
|---|---|
| Avoid using acid blockers and motility agents, such as metoclopramide (Reglan), in premature infants for physiologic gastroesophageal reflux that is effortless and painless, and that does not affect growth. Do not use medication in the so-called “happy spitter.” | American Academy of Pediatrics |
How Should Gastroesophageal Reflux in Premature Infants Be Managed?
Infant positioning and dietary modification, such as smaller, more frequent feedings or a trial of a cow's milk–free diet, should be the initial interventions for gastroesophageal reflux (GER). Although antireflux medications are often used in the immediate neonatal period, there is insufficient evidence of long-term benefit. Antireflux medications may be considered in cases of symptomatic GER not responsive to nonpharmacologic measures.
EVIDENCE SUMMARY
The need to treat GER in premature infants is controversial. The major factor contributing to GER is transient relaxation of the lower esophageal sphincter.6 The daily intake of fluid in infants and horizontal positioning resulting in constant immersion of the gastroesophageal junction strongly suggest that GER is a normal physiologic occurrence. There is little or inconsistent evidence linking GER and cardiorespiratory complications (e.g., chronic lung disease, apnea of prematurity, recurrent aspiration) in premature infants.7,8 Mild physiologic GER must be distinguished from GER disease, which may lead to exacerbation of respiratory symptoms, erosive esophagitis, and growth restriction.
In a retrospective study of 1,598 very low-birth-weight infants, 25% were discharged from the hospital with medications to treat GER. The infants were reevaluated at the 18- to 22-month visit, and the use of antireflux medication was not predictive of growth restriction or neurodevelopmental impairment.9
Nonpharmacologic options are initially preferred for GER and include smaller, more frequent feedings, a trial of thickened feedings (i.e., rice cereal, or newer oligosaccharide-fortified formulas that thicken in the stomach), and infant positioning.10 The U.S. Food and Drug Administration discourages use of xanthan gum thickening agents (e.g., SimplyThick) because they may cause necrotizing enterocolitis.11,12 Feeding should occur in the seated or semi-reclined position to minimize reflux. [ corrected] Placing the infant in the left lateral or prone position after feedings reduces GER symptoms.13 The prone position should be avoided for sleep, however, because it is associated with increased risk of sudden infant death syndrome.14 Additionally, a two-week trial of hypoallergenic formula can be considered for infants with possible cow's milk allergy.
Pharmacologic therapy consists of acid neutralization and prokinetic therapies. Proton pump inhibitors are increasingly used in preterm infants because of improved short- and long-term acid reduction compared with histamine H2 antagonists. Although proton pump inhibitors and H2 antagonists increase the pH of gastric contents, they do not reduce the frequency of reflux symptoms compared with placebo.15,16 There are limited data on the safety and effectiveness of prokinetic agents (e.g., metoclopramide [Reglan], baclofen [Lioresal], erythromycin); therefore, they should not be used routinely.17
Should Exclusively Breastfed Preterm Infants Receive a Multinutrient Fortifier?
Before discharge from the neonatal intensive care unit, supplementation of breast milk with multinutrient fortifiers results in greater short-term weight gain; however, those infants discharged on unfortified breast milk have similar growth to those infants on fortified breast milk at 12 months' corrected age.
EVIDENCE SUMMARY
Weight gain in exclusively breastfed infants tends to be slower than in formula-fed infants.18 Likewise, preterm infants who are fed fortified breast milk before discharge have short-term weight gain, linear growth, and head growth.19 The benefits postdischarge are less clear. A Cochrane review identified two randomized controlled trials (n = 246) comparing infants who were fed fortified breast milk with those who were fed unfortified breast milk for three to four months after discharge. Outcome measures included the various growth parameters. Meta-analysis did not find statistically significant differences in weight or head circumference at 12 months' corrected age; however, infants fed fortified breast milk were longer. This analysis also found evidence that infants fed in response to hunger cues reduced their volume of intake when breast milk was fortified. As a result, these infants on fortified breast milk consumed similar total calories and slightly more protein compared with infants on unfortified breast milk.20
The European Society for Paediatric Gastroenterology, Hepatology, and Nutrition recommends nutrient fortification for exclusively breastfed preterm infants with subnormal weight for corrected age.21
Should Preterm Infants Receive Enriched Formula or Standard Formula Following Hospital Discharge?
Enriched formula is often continued after hospital discharge to avoid extrauterine growth restriction and to promote catch-up growth. However, this practice is not supported by the available evidence.
EVIDENCE SUMMARY
The American Academy of Pediatrics (AAP) found insufficient evidence to make recommendations for the nutritional assessment of the preterm infant after discharge.22,23 The European Society for Paediatric Gastroenterology, Hepatology, and Nutrition recommends that preterm infants with subnormal weight for postconceptual age be given enriched formula until at least 40 weeks' postconceptual age to provide adequate nutrition.21
A Cochrane review found 15 randomized controlled trials (n = 1,128) that compared enriched formula (22 kcal per oz) with standard formula (20 kcal per oz) on growth and development in preterm or low-birth-weight infants following hospital discharge. There was no statistically significant difference in growth parameters up to 12 to 18 months' corrected age. Five trials comparing the use of enriched formula (24 kcal per oz) with standard formula showed evidence of increased growth with enriched formula through 12 to 18 months' corrected age.24
How Can Physicians Promote Appropriate Growth or Catch-Up Growth in Premature Infants After Hospital Discharge?
Premature infants should be weighed biweekly to weekly for the first four to six weeks after hospital discharge, and then every two months thereafter. Nutrient-fortified breast milk or enriched formula should be considered when the infant's weight falls below the 10th percentile for corrected age. Infants who maintain growth or consistently exceed the 10th percentile for corrected age can receive standard formula or unfortified breast milk.
EVIDENCE SUMMARY
The goal of nutritional support in preterm infants is to achieve a postnatal growth rate similar to that of infants at a similar postconceptual age. Despite improvements in neonatal care, approximately 28% of infants born between 23 and 34 weeks' gestation are discharged weighing less than the 10th percentile of expected intrauterine growth (i.e., extrauterine growth restriction).25,26 Neurodevelopmental delay has been associated with inadequate nutrition in the early postnatal period.27
Adequate weight gain of 15 to 20 g per kg per day is difficult to achieve in preterm infants because of higher energy expenditure secondary to prolonged care in the neonatal intensive care unit, illness, and inadequate nutrition. After discharge, a weight gain of 15 to 20 g per kg per day is typically desired for catch-up growth, whereas a gain of 20 to 30 g per kg day is sufficient for those tracking well on the growth curve.28 To achieve these growth rates, the recommended minimal caloric requirement for healthy preterm infants is 110 to 130 kcal per kg per day; however, infants with extrauterine growth restriction may require up to 150 kcal per kg per day.29–31 Growth recovery to the original birth weight percentile should be achieved by one to two months' corrected age.18 The goal for infants with extrauterine growth restriction is to approach the 50th percentile of weight for age by two years' chronologic age. The Fenton growth chart is useful for assessing growth parameters for preterm infants up to 50 weeks' corrected age; thereafter, the World Health Organization growth chart can be used.32 Growth of all preterm infants should be plotted on the growth curve at their corrected age and not their chronologic age until three years of age. The order for catch-up growth is typically head circumference, weight, then length.
Recent evidence has demonstrated that excessive nutrition and weight gain in premature infants may have metabolic effects that increase the risk of developing diabetes mellitus, obesity, chronic hypertension, and cardiovascular disease in adulthood.33,34 Tables 325,35–39 and 440–42 summarize general guidelines for postdischarge nutritional support and anticipated growth velocity.
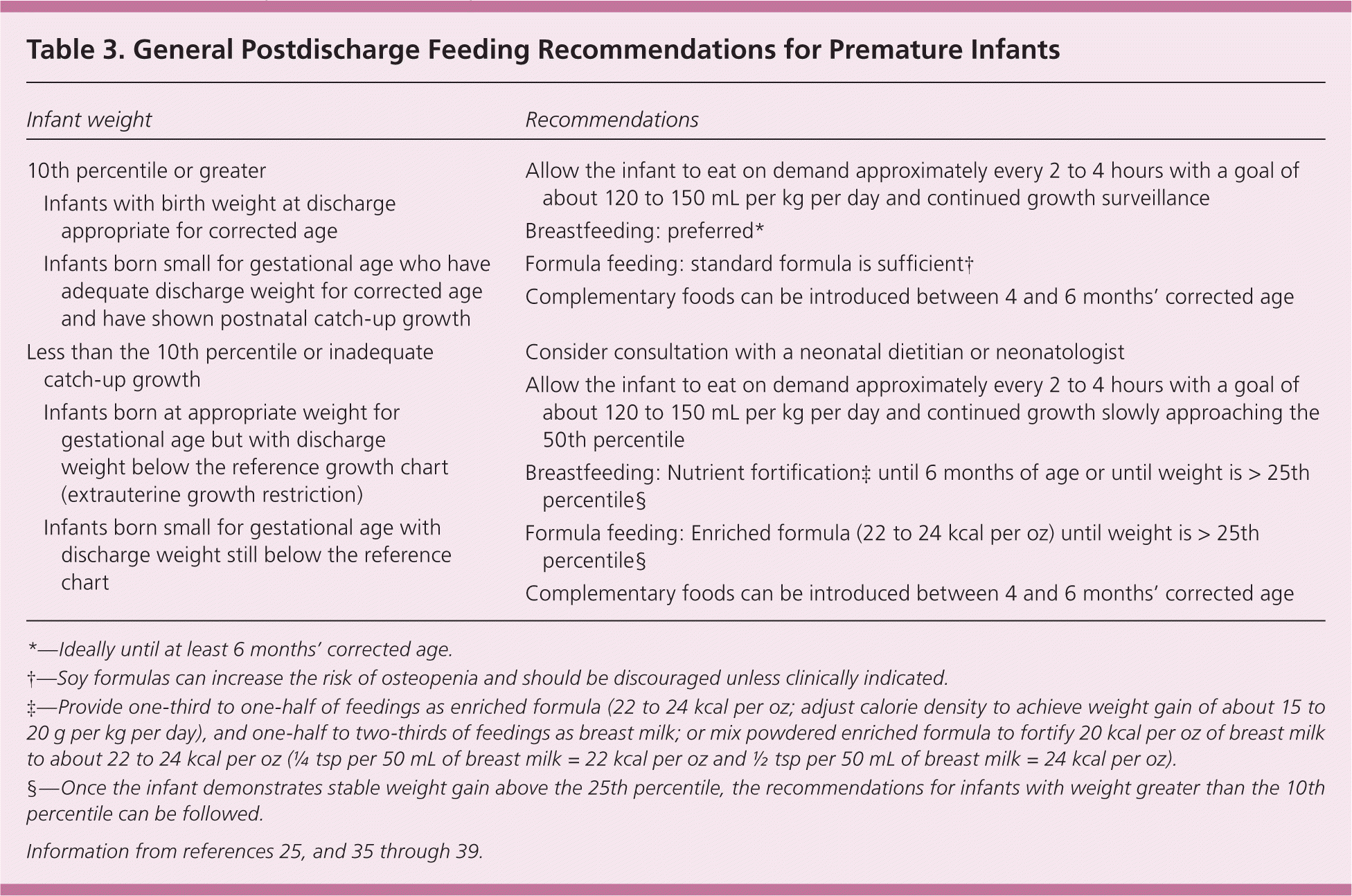
| Infant weight | Recommendations | |
|---|---|---|
| 10th percentile or greater | ||
| Infants with birth weight at discharge appropriate for corrected age | ||
| Infants born small for gestational age who have adequate discharge weight for corrected age and have shown postnatal catch-up growth | ||
| Less than the 10th percentile or inadequate catch-up growth |
| |
| Infants born at appropriate weight for gestational age but with discharge weight below the reference growth chart (extrauterine growth restriction) | ||
| Infants born small for gestational age with discharge weight still below the reference chart | ||
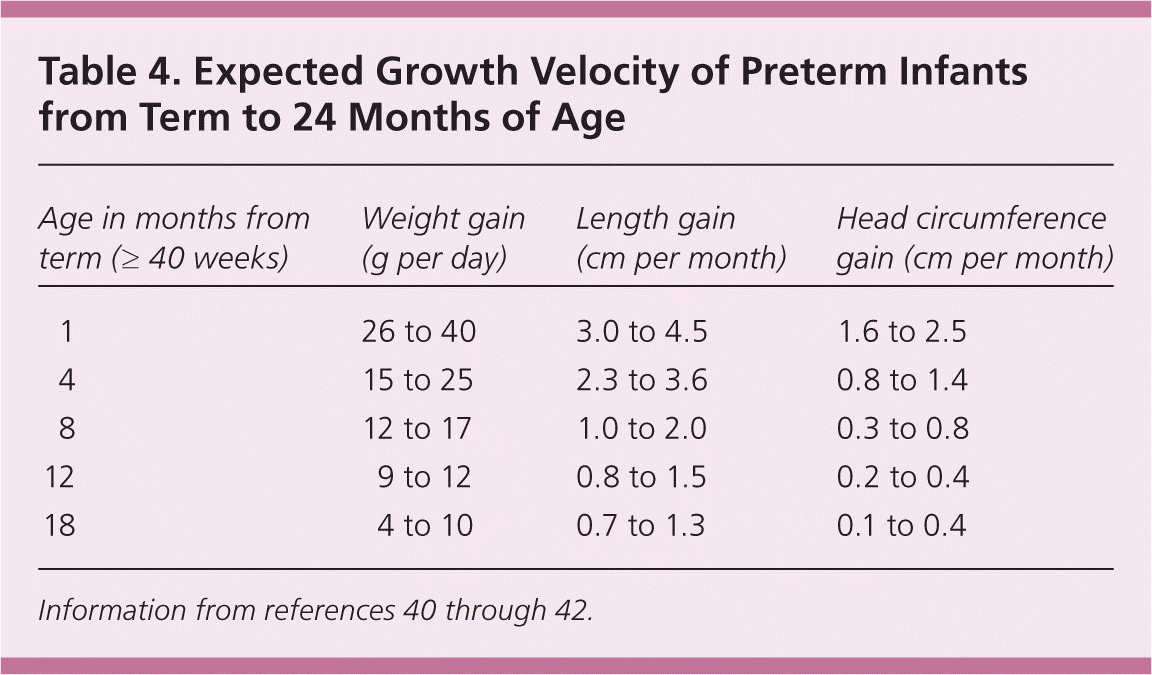
| Age in months from term (≥ 40 weeks) | Weight gain (g per day) | Length gain (cm per month) | Head circumference gain (cm per month) |
|---|---|---|---|
| 1 | 26 to 40 | 3.0 to 4.5 | 1.6 to 2.5 |
| 4 | 15 to 25 | 2.3 to 3.6 | 0.8 to 1.4 |
| 8 | 12 to 17 | 1.0 to 2.0 | 0.3 to 0.8 |
| 12 | 9 to 12 | 0.8 to 1.5 | 0.2 to 0.4 |
| 18 | 4 to 10 | 0.7 to 1.3 | 0.1 to 0.4 |
What Is the Risk of Neurodevelopmental Delay in Premature Infants?
One-half of premature infants develop normally; however, 12% to 50% of very premature infants have disabilities such as cerebral palsy, intellectual disability, and visual or hearing impairment. These infants also later perform worse in mathematics, reading, and spelling compared with term infants. Late preterm infants (born between 34 and 37 weeks' gestation) are nearly four times more likely than term infants to develop cerebral palsy and are at increased risk of functional developmental delays and academic difficulties.
EVIDENCE SUMMARY
A longitudinal study assessed 283 children born at 25 weeks' gestation or earlier for neurologic and developmental disability. They were evaluated at a median of 30 months' corrected age using a detailed medical history, clinical examination, and formal neurologic assessment. Severe developmental, neuromotor, or sensory and communication deficits occured in 23% of patients. Overall, 49% had some degree of disability (95% confidence interval, 43% to 55%).45 Children in this same cohort were reassessed at six years of age and compared with children born full term. Cognitive impairment was the most common disability, occurring in 21% of the study group compared with 1% of the control group. Rates of severe, moderate, and mild disability in the study group were 22%, 24%, and 34%, respectively.46
More than 70% of all preterm births are late preterm births.1,2 These infants may be of similar size to full-term infants and are commonly cared for in normal newborn nurseries. Late preterm infants are at higher risk of adverse neurodevelopmental outcomes.47 In a retrospective cohort study of 141,321 children born at 30 weeks' gestation or later, those born between 34 and 37 weeks' gestation had a nearly fourfold higher risk of cerebral palsy (hazard ratio = 3.7; 95% confidence interval, 2.8 to 4.9) and an increased risk of developmental delay/mental retardation (hazard ratio = 1.4; 95% confidence interval, 1.1 to 1.7) compared with full-term infants.48
The AAP recommends screening for neurodevelopmental delay at nine, 18, and 24 to 30 months of age using a standardized tool (Table 5).49 These tools have a sensitivity and specificity between 70% and 80%.50,51 When a developmental screening tool identifies an infant at risk, a more comprehensive diagnostic evaluation should follow.
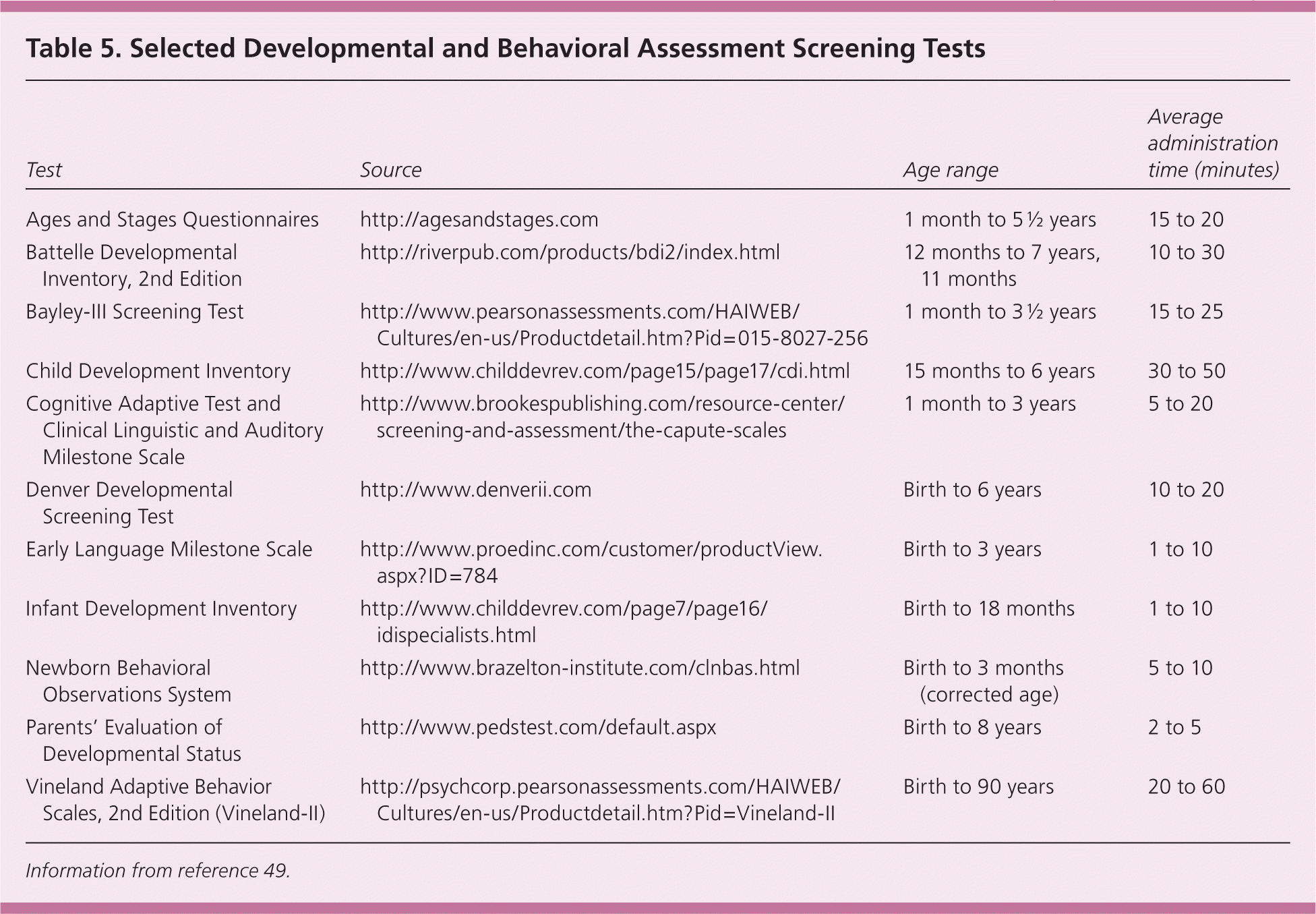
| Test | Source | Age range | Average administration time (minutes) |
|---|---|---|---|
| Ages and Stages Questionnaires | http://agesandstages.com | 1 month to 5 ½ years | 15 to 20 |
| Battelle Developmental Inventory, 2nd Edition | http://riverpub.com/products/bdi2/index.html | 12 months to 7 years, 11 months | 10 to 30 |
| Bayley-III Screening Test | http://www.pearsonassessments.com/HAIWEB/Cultures/en-us/Productdetail.htm?Pid=015-8027-256 | 1 month to 3 ½ years | 15 to 25 |
| Child Development Inventory | http://www.childdevrev.com/page15/page17/cdi.html | 15 months to 6 years | 30 to 50 |
| Cognitive Adaptive Test and Clinical Linguistic and Auditory Milestone Scale | http://www.brookespublishing.com/resource-center/screening-and-assessment/the-capute-scales | 1 month to 3 years | 5 to 20 |
| Denver Developmental Screening Test | http://www.denverii.com | Birth to 6 years | 10 to 20 |
| Early Language Milestone Scale | http://www.proedinc.com/customer/productView.aspx?ID=784 | Birth to 3 years | 1 to 10 |
| Infant Development Inventory | http://www.childdevrev.com/page7/page16/idispecialists.html | Birth to 18 months | 1 to 10 |
| Newborn Behavioral Observations System | http://www.brazelton-institute.com/clnbas.html | Birth to 3 months (corrected age) | 5 to 10 |
| Parents' Evaluation of Developmental Status | http://www.pedstest.com/default.aspx | Birth to 8 years | 2 to 5 |
| Vineland Adaptive Behavior Scales, 2nd Edition (Vineland-II) | http://psychcorp.pearsonassessments.com/HAIWEB/Cultures/en-us/Productdetail.htm?Pid=Vineland-II | Birth to 90 years | 20 to 60 |
Should Premature Infants Be Screened for Iron Deficiency and Iron Deficiency Anemia?
Preterm infants should be screened for anemia at four months of age, with repeat screening between nine and 12 months of age. Breastfed infants should receive 2 mg per kg per day of supplemental iron until complementary foods supply the iron requirement. Infants receiving enriched formula or standard formula receive an adequate amount of fortified iron (approximately 2 mg per kg per day); therefore, the need for additional iron in this group should be based on subsequent screening results.
EVIDENCE SUMMARY
Preterm infants are often anemic because of low iron stores, decreased erythropoietin production, decreased red blood cell survival, infections, and frequent venipuncture. A randomized controlled trial including marginally low-birth-weight infants was conducted to determine if iron supplementation reduced the risk of iron deficiency anemia. In the trial, 285 infants were randomized to receive 0 mg (placebo), 1 mg, or 2 mg per kg per day of supplemental iron between six weeks and six months of age. Secondary end points were growth and associated morbidity. The prevalence of iron deficiency at six months of age was 36% in the placebo group, 8% in the group receiving 1 mg per kg per day of supplemental iron, and 4% in the group receiving 2 mg per kg per day. Rates of iron deficiency anemia in the three groups were 10%, 3%, and 0%, respectively. There were no differences in growth or morbidity. The prevalence of iron deficiency anemia was higher in the placebo group when infants were exclusively breastfed.52
Preterm infants who are breastfed should receive 2 mg per kg per day of supplemental iron from one to 12 months of age or until complementary foods supply the recommended iron requirements.53 Infants who consume 150 mL per kg per day of iron-fortified formula usually do not require additional iron supplementation. However, up to 14% of formula-fed preterm infants develop iron deficiency anemia between four and eight months of age and may require supplementation with 1 mg per kg per day of iron.54
Which Infants Should Receive Respiratory Syncytial Virus Vaccination?
Preterm infants born before 29 weeks' gestation should receive palivizumab (Synagis) prophylaxis in the first year of life to reduce mortality and readmission rates related to respiratory syncytial virus (RSV) infection. Infants born at or after 29 weeks' gestation who are otherwise healthy do not require palivizumab prophylaxis.
EVIDENCE SUMMARY
The hospitalization rate for RSV bronchiolitis in previously discharged premature infants can be up to 37%.55 Preterm infants have significantly higher mortality and readmission rates for RSV compared with full-term infants.56 A randomized, double-blind, placebo controlled trial compared palivizumab injections with placebo in 1,502 premature infants during the RSV season. Prophylaxis resulted in a 55% reduction in RSV-related hospitalization (10.6% placebo vs. 4.8% palivizumab; P < .001).57 A retrospective study showed that the risk of death from RSV was 14 times higher in infants with a birth weight of less than 1,500 g (3 lb, 5 oz) and three times higher in infants with a birth weight of 1,500 to 2,499 g (3 lb, 5 oz to 5 lb, 8 oz), compared with infants with a birth weight greater than 2,499 g.56
The AAP recommends palivizumab in the first year of life during RSV season for all infants born before 29 weeks' gestation and for infants born between 29 and 32 weeks' gestation who have chronic lung disease (defined as more than 21% oxygen requirement for at least the first 28 days of life).58
RSV prophylaxis during the first year of life can also be considered for infants with hemodynamically significant congenital heart disease, pulmonary abnormalities, or neuromuscular disease that impairs clearance of respiratory secretions.58 Prophylaxis during the second year of life is recommended only for infant with profound immunodeficiency or for infants with chronic lung disease who still require chronic medical management.58
Prophylaxis should be initiated at the start of the RSV season (typically between November and March). All infants should receive a maximum of five monthly doses until conclusion of the RSV season. Infants who are eligible for RSV prophylaxis who are born during RSV season may require fewer doses.58
The views expressed herein are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense, or the U.S. government.
Data Sources: We searched OvidSP, Institution for Healthcare Improvement, AHRQ, Cochrane Library, and UpToDate. Key words: prematurity, preterm delivery, epidemiology of prematurity, chronic lung disease, bronchiolitis, respiratory syncytial virus, anemia of prematurity, immunizations, neurodevelopmental delay, neurodevelopmental screening tools, gastroesophageal reflux, postdischarge nutrition, nutritional deficiencies, breastfeeding, palivizumab, iron deficiency anemia, and nutrient fortifiers. Search dates: Nov. 2012 and May 2014.