Am Fam Physician. 2014;90(5):289-296
This is part I of a two-part article on the newborn examination. Part II, “Skin, Trunk, Extremities, Neurologic,” appears in this issue of AFP.
Author disclosure: No relevant financial affiliations.
A comprehensive newborn examination involves a systematic inspection. A Ballard score uses physical and neurologic characteristics to assess gestational age. Craniosynostosis is caused by premature fusion of the sutures, and 20% of children with this condition have a genetic mutation or syndrome. The red reflex assessment is normal if there is symmetry in both eyes, without opacities, white spots, or dark spots. If the red reflex findings are abnormal or the patient has a family history of pertinent eye disorders, consultation with an ophthalmologist is warranted. Newborns with low-set ears should be evaluated for a genetic condition. Renal ultrasonography should be performed only in patients with isolated ear anomalies, such as preauricular pits or cup ears, if they are accompanied by other malformations or significant family history. If ankyloglossia is detected, a frenotomy may be considered if it impacts breastfeeding. The neck should be examined for full range of motion because uncorrected torticollis can lead to plagiocephaly and ear misalignment. Proper auscultation is crucial for evaluation of the broncho-pulmonary circulation with close observation for signs of respiratory distress, including tachypnea, nasal flaring, grunting, retractions, and cyanosis. Benign murmurs are often present in the first hours of life. Pulse oximetry should be performed in a systematic fashion before discharge.
Part I of this two-part article discusses the assessment of general health, head and neck, heart, and lungs. Part II focuses on assessing extremities, and neurologic function.1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Screening for hypoglycemia should be performed in newborns who are large or small for gestational age, newborns of mothers with diabetes mellitus, and late preterm infants (34 to 36 6/7 weeks gestational age). | C | 7 |
| Regardless of red reflex test results, all newborns with a family history of retinoblastoma, cataracts, glaucoma, or retinal abnormalities should be referred to an ophthalmologist who is experienced in the examination of children. | C | 17 |
| Hearing should be evaluated in all newborns before one month of age, but preferably before discharge, using the auditory brainstem response or the otoacoustic emissions test. | C | 20 |
| Recent data indicate that ultrasonography should be performed in newborns with isolated ear anomalies, such as preauricular pits or cup ears, only when they are associated with one or more of the following characteristics: other malformations or dysmorphic features, teratogenic exposures, a family history of deafness, or a maternal history of gestational diabetes. | C | 26, 27 |
| Routine screening for congenital heart disease via pulse oximetry is recommended before discharge at 24 hours of life or later. Diagnostic echocardiography should be performed if screening results are positive. | C | 40 |
General Assessment
A detailed newborn examination should begin with general observation for normal and dysmorphic features. A term newborn should have pink skin, rest symmetrically with the arms and legs in flexion, cry vigorously when stimulated, and move all extremities equally. Table 1 shows the normal ranges for newborn vital signs at 40 weeks' gestation.2–4 The new Ballard score (http://www.ballardscore.com) was designed to assess a newborn's gestational age through a scoring system that combines physical characteristics with neuromuscular development.5 A video depicting this examination is available at http://www.ballardscore.com/Pages/videos.aspx. Once the child's gestational age is established, weight, length, and head circumference should be plotted on a nomogram to determine percentiles. Using this information, the newborn can be classified as average, large, or small for gestational age.
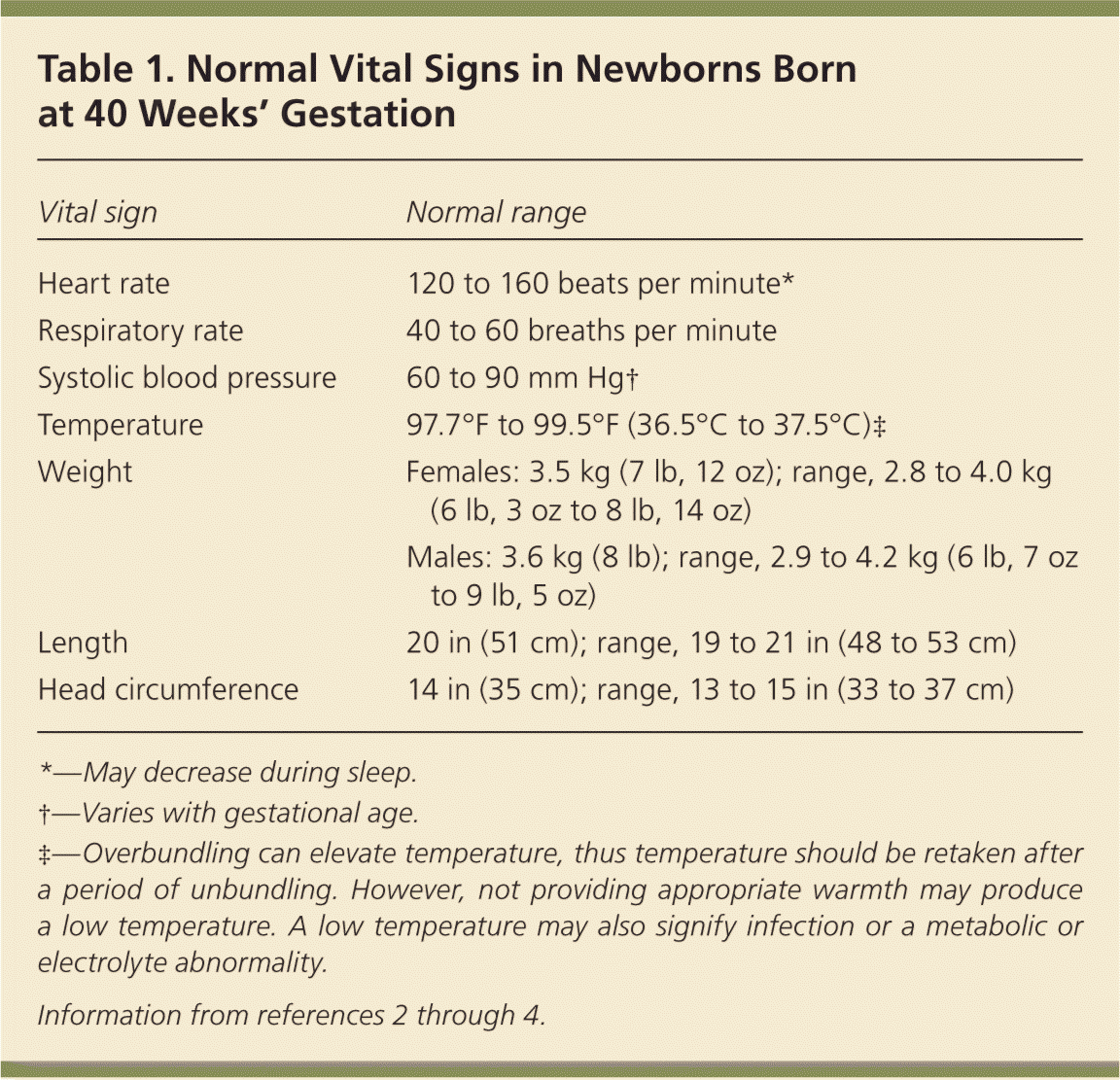
| Vital sign | Normal range |
|---|---|
| Heart rate | 120 to 160 beats per minute* |
| Respiratory rate | 40 to 60 breaths per minute |
| Systolic blood pressure | 60 to 90 mm Hg† |
| Temperature | 97.7°F to 99.5°F (36.5°C to 37.5°C)‡ |
| Weight | Females: 3.5 kg (7 lb, 12 oz); range, 2.8 to 4.0 kg (6 lb, 3 oz to 8 lb, 14 oz) |
| Males: 3.6 kg (8 lb); range, 2.9 to 4.2 kg (6 lb, 7 oz to 9 lb, 5 oz) | |
| Length | 20 in (51 cm); range, 19 to 21 in (48 to 53 cm) |
| Head circumference | 14 in (35 cm); range, 13 to 15 in (33 to 37 cm) |
A newborn is considered small for gestational age if birth weight is below the 10th percentile. Intrauterine growth restriction occurs when the baby's growth during pregnancy is poor compared with norms. Measurements that are symmetrically decreased suggest that the newborn has a chronic exposure (e.g., maternal smoking or drug use) that impacted growth, or a congenital infection such as a TORCH infection (toxoplasmosis, other agents, rubella, cytomegalovirus, herpes simplex), a metabolic disorder, or a chromosomal abnormality (e.g., Turner syndrome, trisomies). Newborns with these conditions often display dysmorphic features or are simply constitutionally small. If the causative factor occurred later in pregnancy (e.g., uteroplacental insufficiency), the head circumference will be preserved relative to other measurements.6 A newborn with a birth weight above the 90th percentile is considered large for gestational age. The most common cause is maternal diabetes mellitus, although other causes include a metabolic or genetic syndrome such as Beckwith-Wiedemann syndrome.
Because of an increased risk of hypoglycemia, the American Academy of Pediatrics recommends scheduled glucose screening for newborns who are large or small for gestational age, newborns of mothers with diabetes, and late preterm newborns (34 to 36 6/7 weeks gestational age), and provides protocols for their management.7
Head
At 40 weeks' gestation, the average head circumference is 14 in (35 cm); range, 13 to 15 in (33 to 37 cm, 10th to 90th percentile).2 Microcephaly (isolated asymmetrically small head, less than the second percentile or two standard deviations below the mean for age and sex) may indicate central nervous system malformation (e.g., holoprosencephaly, neural tube defect), an infection (e.g., toxoplasmosis, cytomegalovirus infection), or a genetic syndrome (e.g., trisomy 13 and 18 syndrome, fetal alcohol syndrome). Macrocephaly (isolated head enlargement, greater than the 98th percentile or greater than two standard deviations above the mean) may be hereditary or the result of a central nervous system disorder (e.g., hydrocephalus, brain tumor), and imaging may be needed.3,4
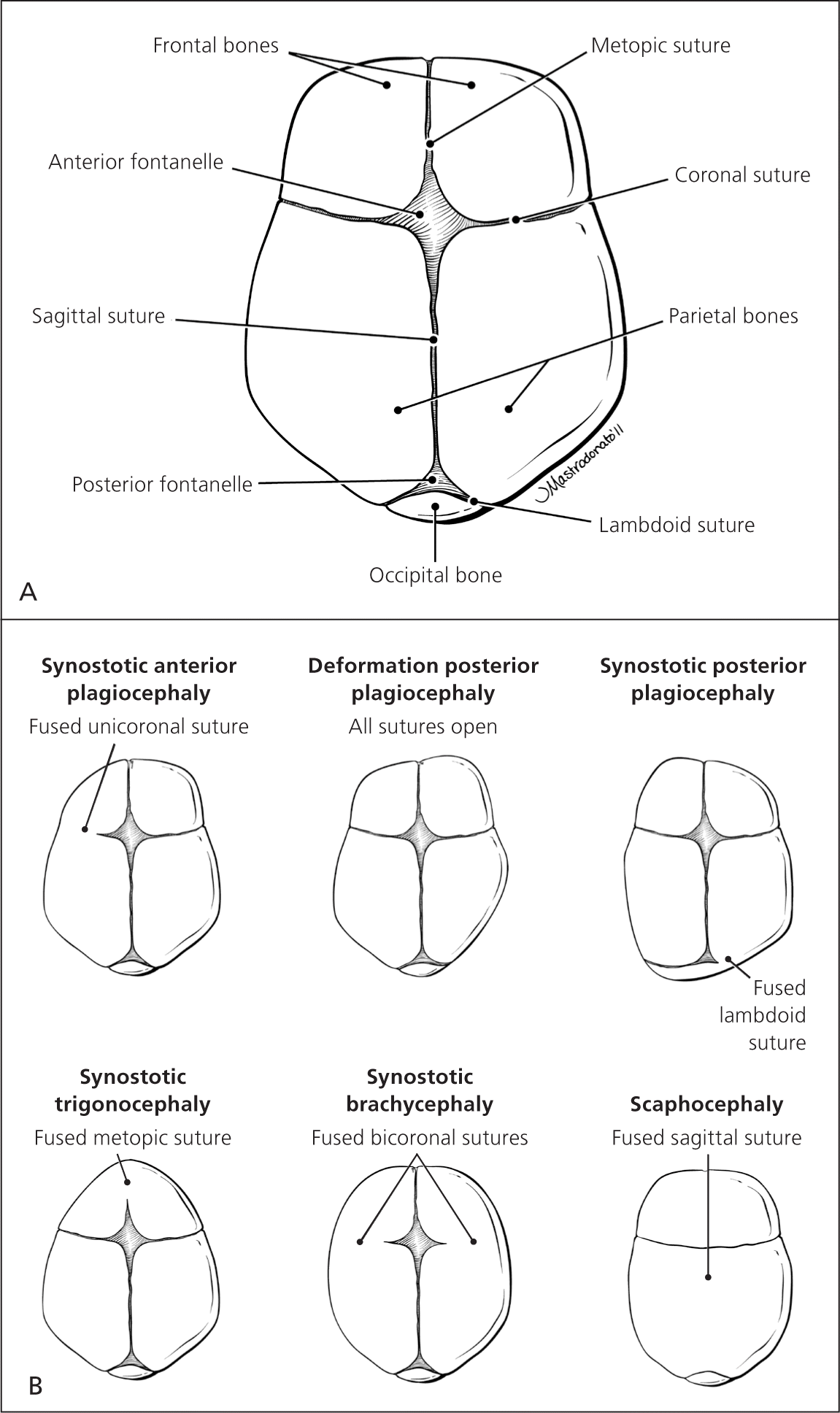
After evaluating the overall size and shape of the head for asymmetry or gross structural abnormalities, the fontanelles and sutures should be palpated with the newborn in the upright position. Figure 1 illustrates a normal newborn skull and common deformities. The anterior fontanelle is generally 3 to 6 cm in diameter, whereas the posterior fontanelle is no larger than 1 to 1.5 cm in diameter. A large anterior fontanelle may indicate increased intracranial pressure, Down syndrome, hypophosphatemia, trisomy, or congenital hypothyroidism. Fontanelles are often small in newborns with microcephaly. A prematurely fused suture indicates craniosynostosis and occurs in one out of 1,000 newborns.8,9 Craniosynostosis limits growth of the skull in a direction perpendicular to the suture, while growth may continue in other directions. More than 20% of cases are caused by specific single-gene mutations or chromosomal abnormalities and may be associated with conditions such as Crouzon, Apert, and Pfeiffer syndromes.10 A misshapen head may be caused by prenatal compressions rather than true synostosis. If this is the case, the misshapen head should resolve spontaneously within the first few months of life.11
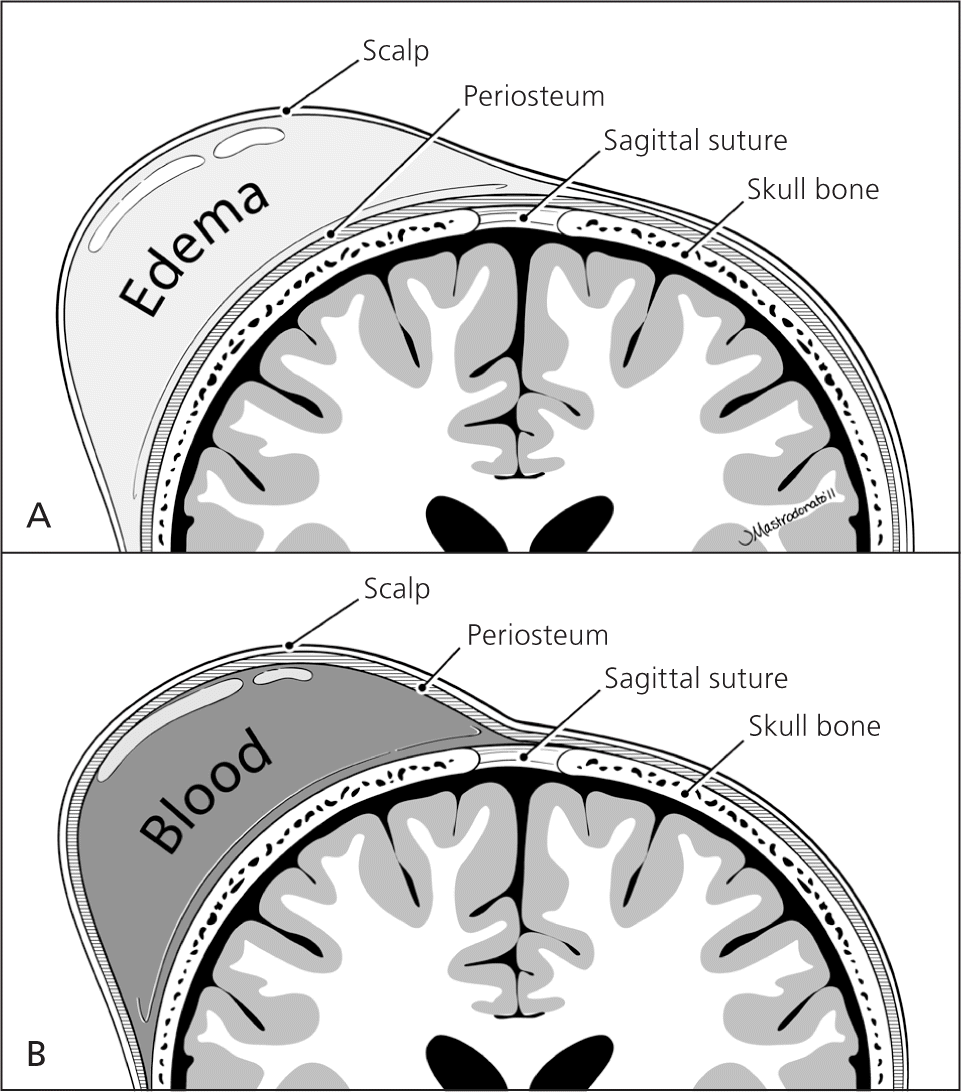
The scalp examination may reveal caput succedaneum, cephalohematoma, and other lesions (Figure 2). A caput succedaneum is scalp edema that is not limited by suture lines, is often pitting, and decreases over time. Most caputs resolve within 48 hours. A cephalohematoma is caused by injury of a blood vessel in the subperiosteal layer of the calvaria. It is limited by suture lines and occurs more commonly in deliveries in which forceps or a vacuum extractor was used. Cephalohematoma is a risk factor for jaundice and sepsis and may worsen over 48 hours, potentially taking up to three to four months to fully reabsorb. Skull fractures are rarely present. If a fracture is depressed or accompanied by neurologic symptoms, computed tomography should be performed to rule out intracranial pathology.12
Forceps use or a difficult delivery may also lead to a facial nerve palsy resulting in the inability to close the eye, loss of the nasolabial fold, drooping at the corner of mouth, or the inability to contract the ipsilateral lower facial muscles. This usually resolves within the first few weeks of life, but further evaluation is warranted if symptoms persist.13
Eyes
The newborn evaluation should include noting eye color; pupil size; appearance of the conjunctiva, sclera, and eyelid; eye movement; and spacing between the eyes. Genetic syndromes often cause unusual eye shape, such as epicanthal folds (excess skin over the medial aspect of the eye) and upslanting of palpebral fissures associated with Down syndrome. Colobomas (a gap or defect in the structure of the eye, primarily the iris) may occur with many syndromes, including CHARGE (coloboma of the eye, heart defects, choanal atresia, retraction of growth and/or development, genital and/or urinary abnormalities, and ear abnormalities and deafness). Infants with colobomas need a formal ophthalmology evaluation. Hypertelorism (increased space between the eyes) and hypotelorism (decreased space between the eyes) are often associated with a genetic disorder.14 The visual acuity of newborns is approximately 20/400, and a dysconjugate gaze is normal in the first two to three months of life. Subconjunctival hemorrhages from blood vessel rupture are also a common benign finding that may take weeks to resolve.
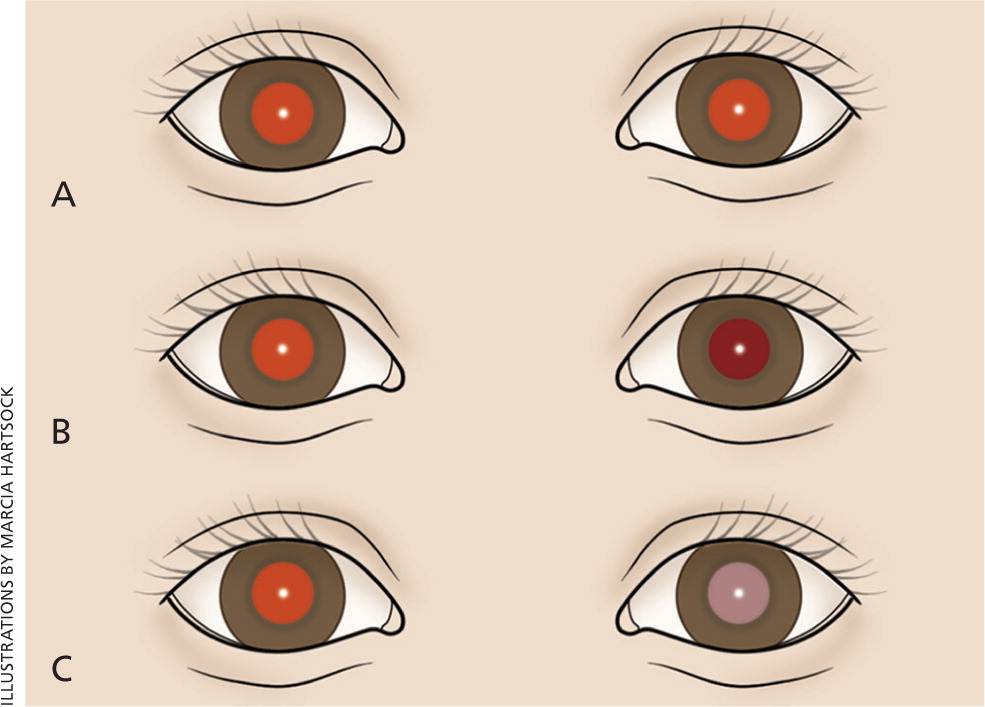
The red reflex test is performed by using an ophthalmoscope, with the lens power set at 0 and the examiner standing approximately 18 inches away. Light should project onto both eyes simultaneously. A red reflex result is normal if there is symmetry in both eyes without opacities, white spots, or dark spots (Figure 315 ). The color of the reflex may be different among ethnic groups because of varying amounts of pigmentation in the ocular fundus; however, the reflex should not be white.14,16 Table 2 gives a differential diagnosis of leukokoria.14,16 An abnormal red reflex result warrants urgent referral to an ophthalmologist. Regardless of red reflex findings, all newborns with a family history of retinoblastoma, cataracts, glaucoma, or retinal abnormalities should be referred to an ophthalmologist experienced in the examination of children because of the high risk of serious eye abnormalities.17
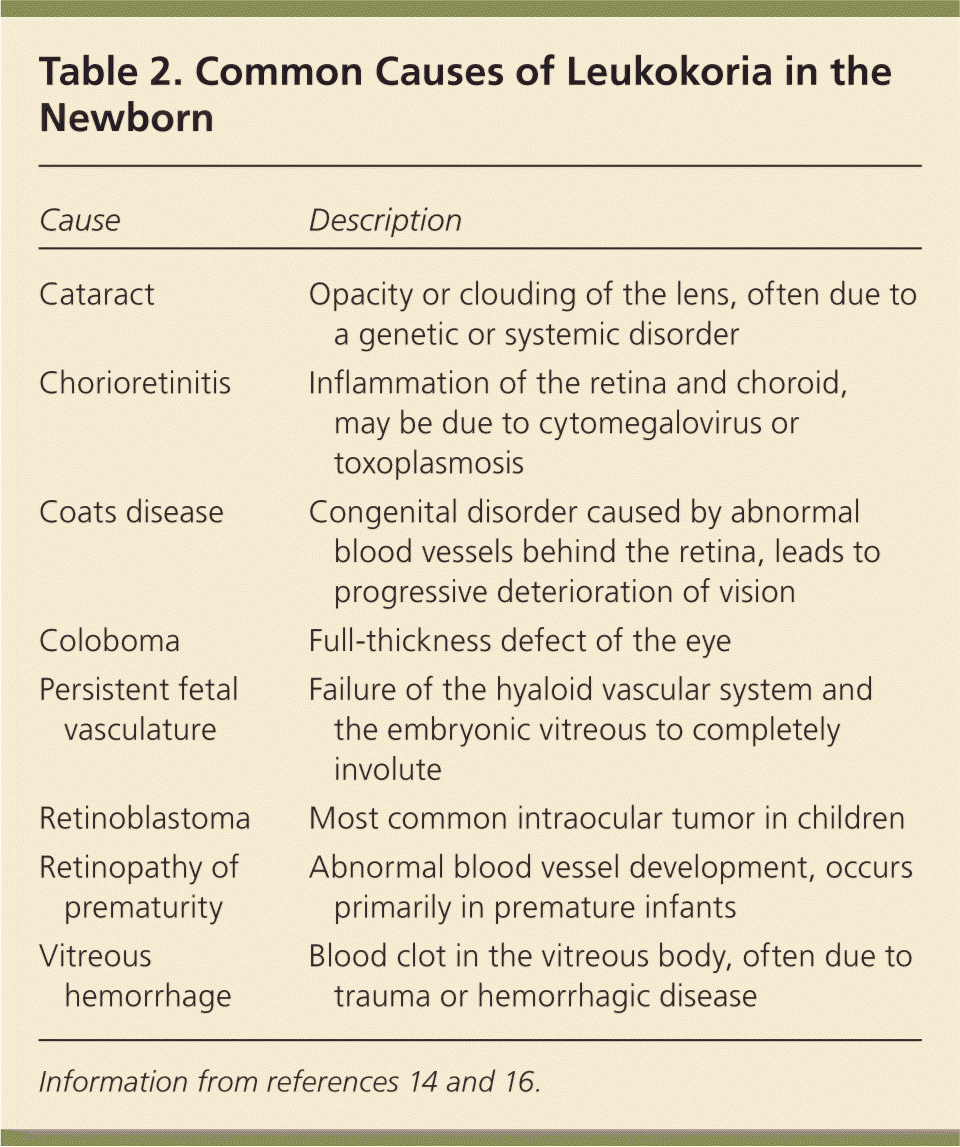
| Cause | Description |
|---|---|
| Cataract | Opacity or clouding of the lens, often due to a genetic or systemic disorder |
| Chorioretinitis | Inflammation of the retina and choroid, may be due to cytomegalovirus or toxoplasmosis |
| Coats disease | Congenital disorder caused by abnormal blood vessels behind the retina, leads to progressive deterioration of vision |
| Coloboma | Full-thickness defect of the eye |
| Persistent fetal vasculature | Failure of the hyaloid vascular system and the embryonic vitreous to completely involute |
| Retinoblastoma | Most common intraocular tumor in children |
| Retinopathy of prematurity | Abnormal blood vessel development, occurs primarily in premature infants |
| Vitreous hemorrhage | Blood clot in the vitreous body, often due to trauma or hemorrhagic disease |
Dacryostenosis should be differentiated from ophthalmia neonatorum, which is conjunctivitis within the first four weeks of life (Table 3).18 With dacryostenosis, a blocked tear duct causes secretions to accumulate with a yellow sticky appearance while the rest of the eye appears normal.19 With conjunctivitis, however, there is often edema and conjunctival injection.18
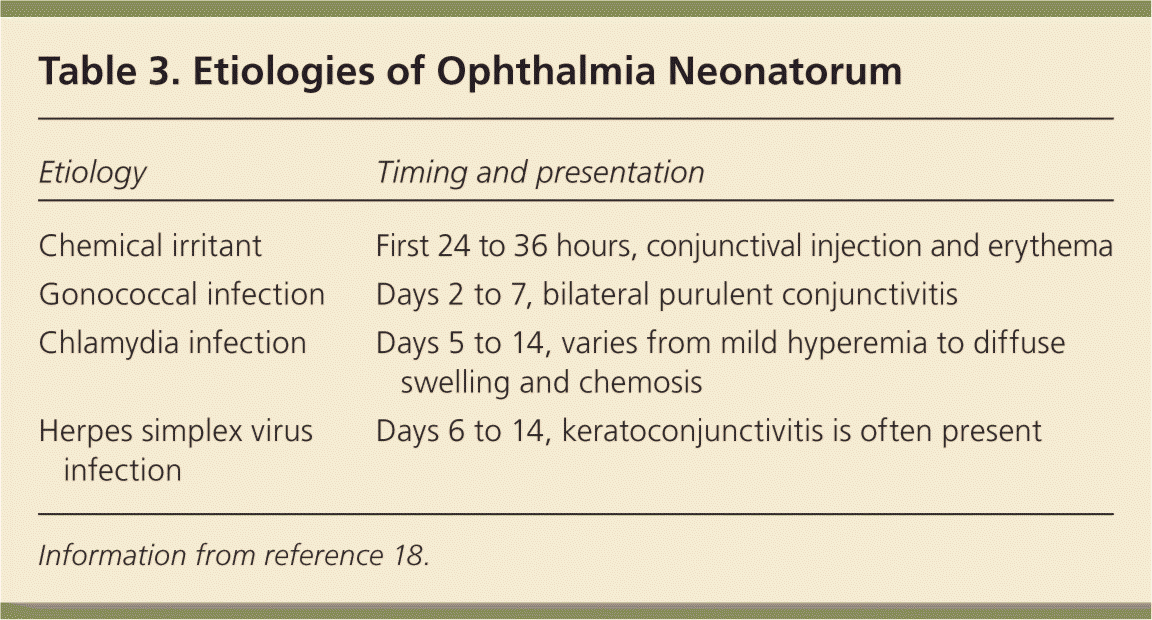
| Etiology | Timing and presentation |
|---|---|
| Chemical irritant | First 24 to 36 hours, conjunctival injection and erythema |
| Gonococcal infection | Days 2 to 7, bilateral purulent conjunctivitis |
| Chlamydia infection | Days 5 to 14, varies from mild hyperemia to diffuse swelling and chemosis |
| Herpes simplex virus infection | Days 6 to 14, keratoconjunctivitis is often present |
Ears
Hearing should be evaluated in all newborns before one month of age, but preferably before discharge, using the auditory brainstem response or the otoacoustic emissions test.20 Assessing the size, shape, and position of the ears may reveal congenital abnormalities. Ears are considered low-set when the helix of the ear meets the cranium at a level below that of a horizontal plane through both inner canthi (Figure 4). Low-set ears are often a sign of a genetic condition (e.g., Down, Turner, or trisomy 18 syndrome). Microtia (small and underdeveloped pinnae) is commonly associated with another defect, such as CHARGE syndrome.21 Because preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns, screening and close monitoring are warranted.22 There is a known association between ear and renal abnormalities, and a variety of syndromes demonstrate both ear and renal defects.23–25
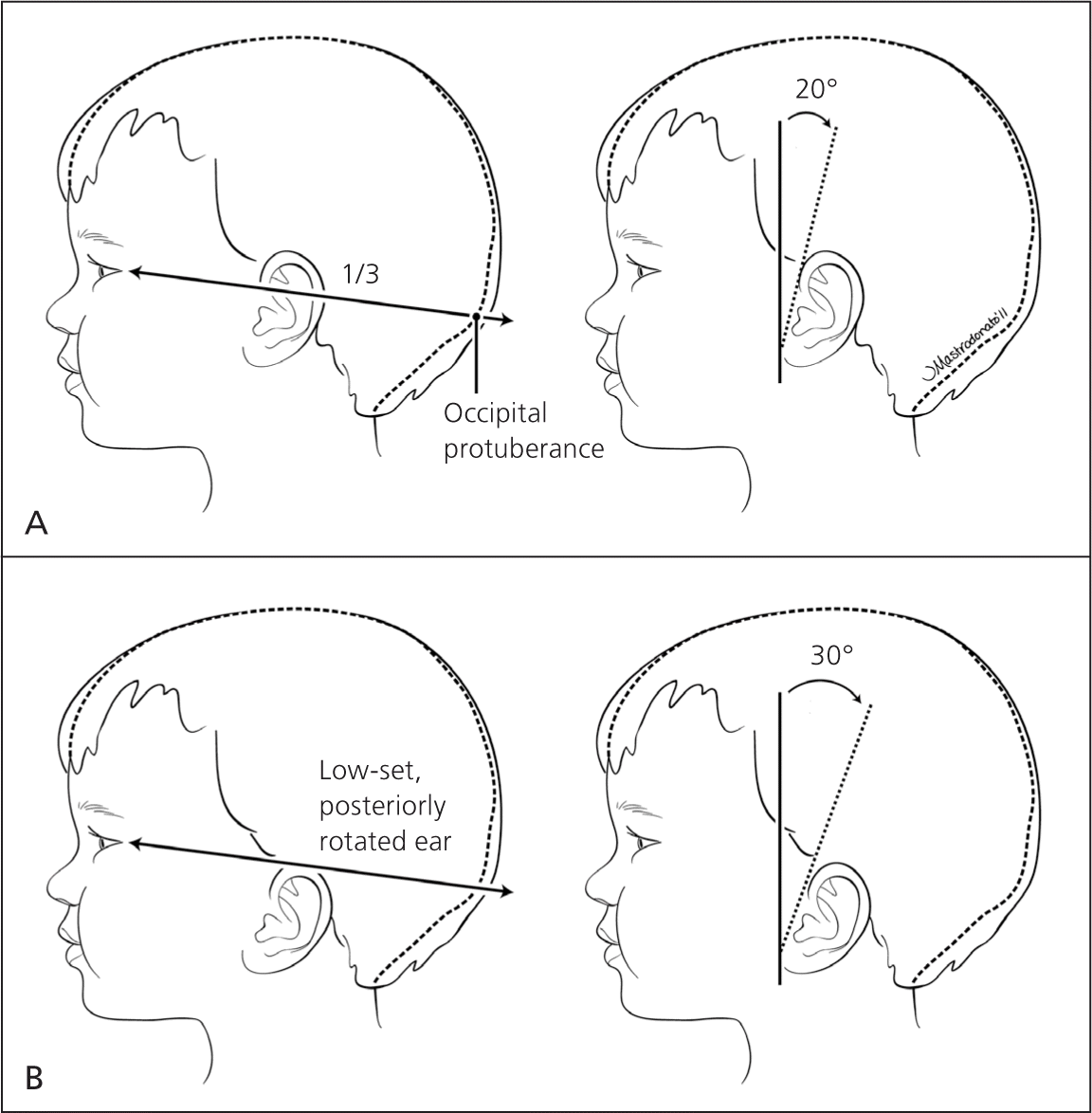
In the past, there was uncertainty about which ear malformations warranted screening renal ultrasonography. Recent data indicate that ultrasonography should be performed in patients with isolated ear anomalies, such as preauricular pits or cup ears, only when they are associated with one or more of the following characteristics: other malformations or dysmorphic features, teratogenic exposures, a family history of deafness, or a maternal history of gestational diabetes.26–28 Ear canals should be observed for patency.
Nose
Choanal atresia occurs when one or both sides of the nasal airway are narrowed or blocked. To assess patency of the nostrils, a small-caliber catheter can be passed through the nasal passages. If bilateral choanal atresia is present, the infant may have cyanosis that is relieved by crying. Asymmetry of the nasal septum is often due to in utero positioning. If it can be corrected by depression of the tip of the nose, it will usually resolve on its own. However, asymmetry that does not correct with depression of the nose tip indicates a dislocated septum, and the patient should be evaluated by an otolaryngologist.29,30
Mouth
The maxilla and mandible should fit together well and open at equal angles. Micrognathia (a small mandible) occurs with Pierre Robin syndrome. Table 4 details common oral cavity findings.31 Ankyloglossia occurs when a short frenulum attaches the tongue to the floor of the mouth, limiting its mobility. This may interfere with breastfeeding or impair articulation, although frenotomy is controversial.32–34 Palpating the palate can reveal submucosal and mucosal clefts. A bifid uvula is often associated with a submucosal cleft. Cleft lip and palate are the most common anomalies of the head and neck. Midline clefts warrant investigation for a midline defect in the brain or other abnormalities.35

| Finding | Description |
|---|---|
| Bohn nodules | Remnants of salivary gland tissue on the lateral aspect of the gum, resolve spontaneously |
| Epstein pearls | White cystic vesicles (1 to 3 mm) on the median palatal raphe of the mouth, oral counterpart to milia, resolve spontaneously |
| Natal teeth* | Often occur on the lower gum, should be removed if loose because of aspiration risk |
| Ranula | Mucus retention cysts on the floor of mouth, often require surgical removal |
Neck
The neck should be inspected for full range of motion because congenital torticollis is a common musculoskeletal anomaly of newborns. Torticollis is primarily due to birth trauma to the sternocleidomastoid muscle that causes swelling or sometimes hematoma formation within the muscle. It can usually be corrected with physical therapy.36 If not corrected, torticollis can lead to plagiocephaly and ear misalignment.
Other possible findings on the neck examination include webbing, which can occur with Turner syndrome, and branchial clefts, pits, and masses. A cystic hygroma is a congenital lymphatic malformation in the neck region. A midline neck lesion may represent a thyroglossal duct cyst and typically shifts with movement of the tongue. The clavicles should be palpated for fracture, which may manifest only as asymmetric Moro reflex if nondisplaced. A suspected fracture should be confirmed with a radiograph. Fractures can be a result of birth trauma and are typically treated with analgesics for pain.37
Heart
When a newborn takes the first breath, subsequent decreases in resistance in the pulmonary vasculature and increases in oxygen concentration result in eventual closure of the shunts, which allow the newborn to transition to adult circulation. Congenital heart disease occurs in approximately six out of 1,000 live births.38 Newborns with heart disease often exhibit tachypnea without retractions. Cyanosis is often present with severe disease.38 This appearance should be differentiated from acrocyanosis (isolated cyanosis of the hands and feet), which is normal in newborns.
Upon auscultation of the heart in the standard four locations (right upper sternal border, left upper sternal border, left lower sternal border, and between the fifth and sixth intercostal space in the midclavicular line), the first heart sound should be single and the second heart sound split. Table 5 summarizes important cardiac examination findings.38,39 Because of changes in vasculature immediately after birth, benign murmurs are common in the first hours of life.38,39 Routine screening for congenital heart disease via pulse oximetry is recommended before discharge at 24 hours of life or later, or shortly before discharge if earlier than 24 hours. Diagnostic echocardiography should be performed if screening results are positive (Table 6).40,41
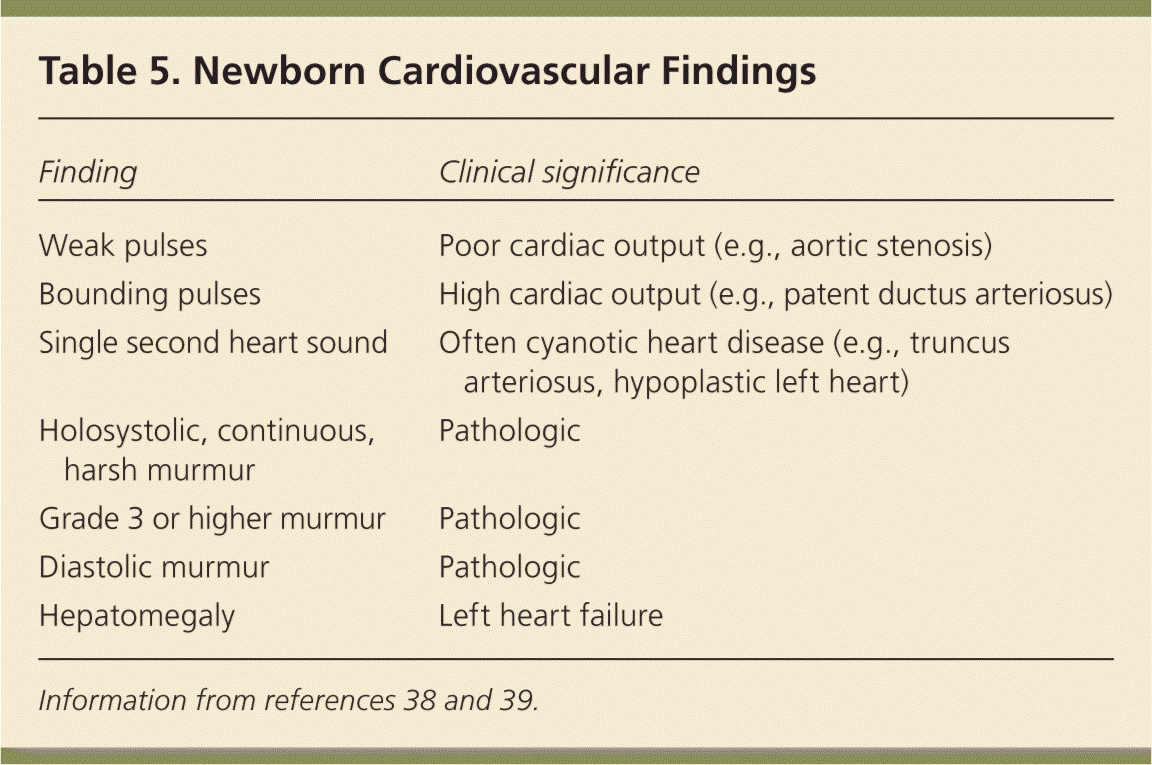
| Finding | Clinical significance |
|---|---|
| Weak pulses | Poor cardiac output (e.g., aortic stenosis) |
| Bounding pulses | High cardiac output (e.g., patent ductus arteriosus) |
| Single second heart sound | Often cyanotic heart disease (e.g., truncus arteriosus, hypoplastic left heart) |
| Holosystolic, continuous, harsh murmur | Pathologic |
| Grade 3 or higher murmur | Pathologic |
| Diastolic murmur | Pathologic |
| Hepatomegaly | Left heart failure |
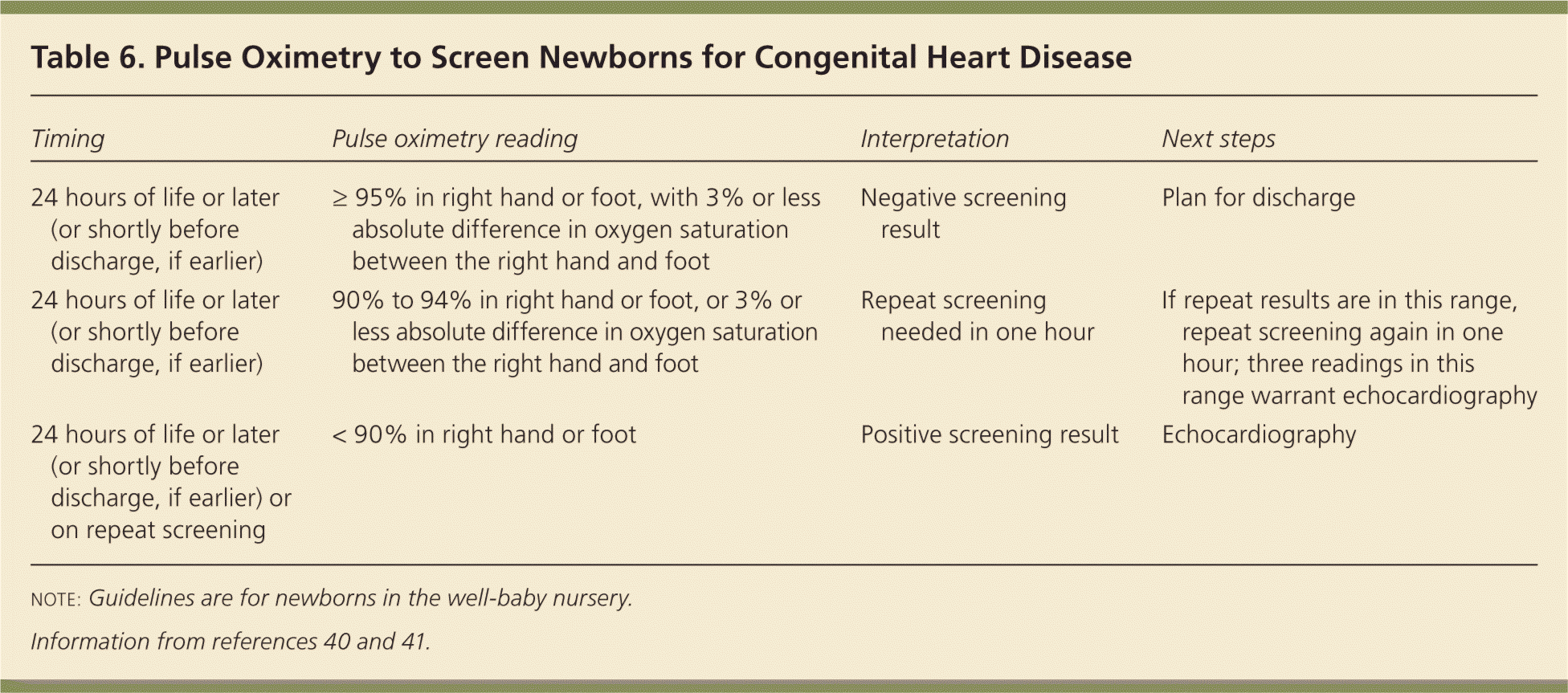
| Timing | Pulse oximetry reading | Interpretation | Next steps |
|---|---|---|---|
| 24 hours of life or later (or shortly before discharge, if earlier) | ≥ 95% in right hand or foot, with 3% or less absolute difference in oxygen saturation between the right hand and foot | Negative screening result | Plan for discharge |
| 24 hours of life or later (or shortly before discharge, if earlier) | 90% to 94% in right hand or foot, or 3% or less absolute difference in oxygen saturation between the right hand and foot | Repeat screening needed in one hour | If repeat results are in this range, repeat screening again in one hour; three readings in this range warrant echocardiography |
| 24 hours of life or later (or shortly before discharge, if earlier) or on repeat screening | < 90% in right hand or foot | Positive screening result | Echocardiography |
Lungs
The respiratory examination is important because the infant is transitioning from fetal to neonatal life. The alveoli are filling with air, the systemic vascular resistance is increasing, and the pulmonary vascular resistance is decreasing. The examiner should observe for signs of respiratory distress, including tachypnea, nasal flaring, grunting, retractions, and cyanosis. Breath sounds should be equal on auscultation. Unequal breath sounds may indicate a pneumothorax and should prompt imaging.
Transient tachypnea of the newborn occurs predominantly in those born via cesarean delivery or precipitous delivery. It is caused by retained fluid in the lungs, which can result in alveolar hypoventilation.42 Treatment includes supportive respiratory care because the condition resolves within 48 hours. Respiratory distress syndrome arises from lack of surfactant, which leads to alveolar collapse. Although it is most common in preterm infants, it may occur in term infants, particularly if the mother has diabetes. A previous article in American Family Physician includes a detailed review of respiratory distress in the newborn.43
Data Sources: A PubMed search was completed using the terms infant, newborn, developmental delay, developmental disturbance, and physical examination. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched POEMs (patient-oriented evidence that matters), Clinical Evidence, the Cochrane database, and Essential Evidence Plus. Search dates: January 1, 2012, and May 2, 2014.
The opinions and assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army Service at large.