
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2014;90(6):377-382
A more recent article on health care–associated infections is available.
Author disclosure: No relevant financial affiliations.
Health care–associated infections cause approximately 75,000 deaths annually, in addition to increasing morbidity and costs. Over the past decade, a downward trend in health care–associated infections has occurred nationwide. Basic prevention measures include administrative support, educating health care personnel, and hand hygiene and isolation precautions. Prevention of central line– or catheter-associated infections begins with avoidance of unnecessary insertion, adherence to aseptic technique when inserting, and device removal when no longer necessary. Specific recommendations for preventing central line–associated bloodstream infections include use of chlorhexidine for skin preparation, as a component of dressings, and for daily bathing of patients in intensive care units. Catheter-associated urinary tract infections are the most common device-related health care–associated infection. Maintaining a closed drainage system below the patient reduces the risk of infection. To prevent ventilator-associated pneumonia, which is associated with high mortality, mechanically ventilated patients should be placed in the semirecumbent position and receive antiseptic oral care. Prevention of surgical site infections includes hair removal using clippers, glucose control, and preoperative antibiotic prophylaxis. Reducing transmission of Clostridium difficile and multidrug-resistant organisms in the hospital setting begins with hand hygiene and contact precautions. Institutional efforts to reduce unnecessary antibiotic prescribing are also strongly recommended. Reducing rates of methicillin-resistant Staphylococcus aureus infection can be achieved through active surveillance cultures and decolonization therapy with mupirocin.
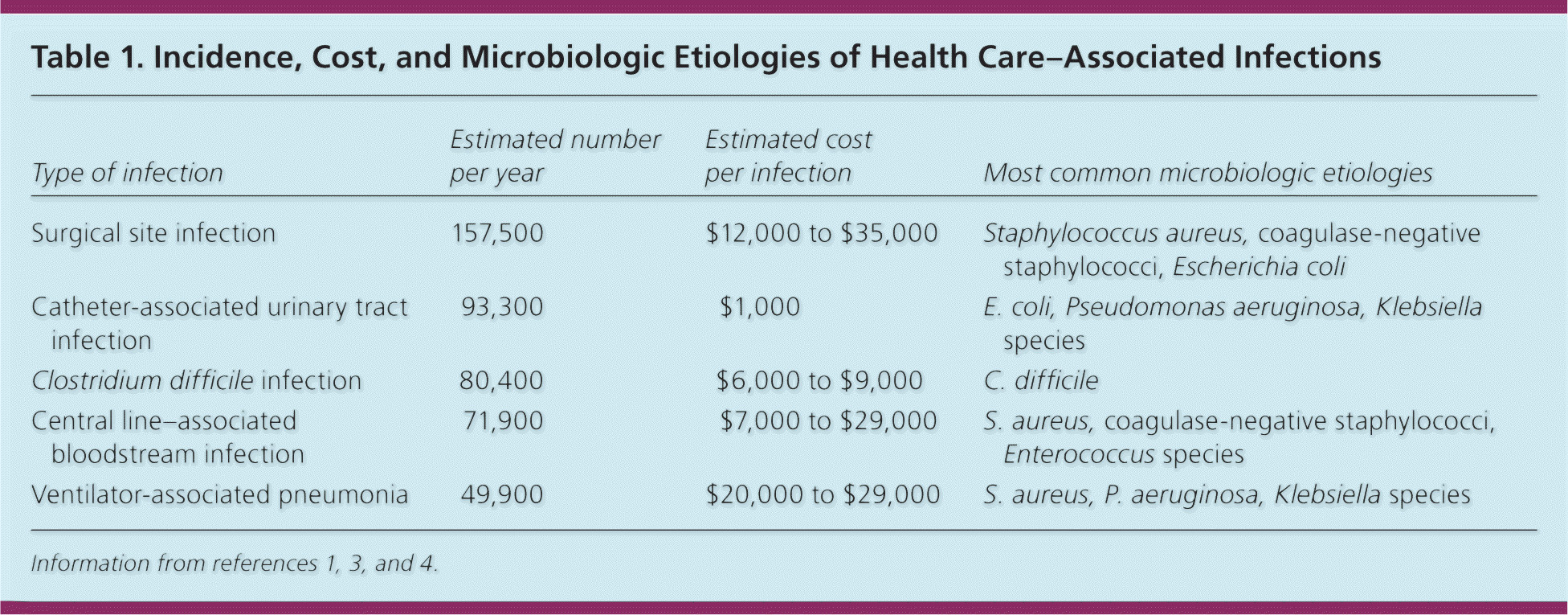
Health care–associated infections are a leading cause of morbidity and mortality among hospitalized patients. Although significant progress has been made in reducing the burden of disease, more than 700,000 such infections and approximately 75,000 deaths occur annually, which if counted as a separate category would represent the seventh leading cause of death in the United States.1,2 Health care–associated infections can be broadly classified into device-, procedure-, and antibiotic-associated infections. In addition to the direct impact on patient outcomes, these infections increase length of hospital stay and health care costs (Table 1).1,3,4
| Clinical recommendation | Evidence rating | References | ||
|---|---|---|---|---|
| To prevent central line–associated bloodstream infections: | A | 5, 19–24 | ||
| Before and during insertion: | ||||
| Avoid the femoral vein for central venous catheter insertion, if possible. | ||||
| Use sterile barrier precautions. | ||||
| Prepare skin with chlorhexidine. | ||||
| Apply a chlorhexidine-impregnated dressing. | ||||
| After insertion: | ||||
| Bathe patients in intensive care units daily with chlorhexidine. | ||||
| Promptly remove nonessential intravascular catheters. | ||||
| To prevent catheter-associated urinary tract infections: | C | 6, 26 | ||
| Insert a urinary catheter only if necessary, and leave in place only as long as needed. | ||||
| Maintain a closed, unobstructed drainage system below the level of the bladder at all times. | ||||
| Change the catheter if obstruction occurs. | ||||
| To prevent ventilator-associated pneumonia: | A | 7, 8, 29, 31–33 | ||
| Promote noninvasive positive pressure ventilation. | ||||
| Keep mechanically ventilated patients in the semirecumbent position rather than supine. | ||||
| Perform regular antiseptic oral care. | ||||
| To prevent surgical site infections: | C | 9, 34–38 | ||
| Remove hair preoperatively only if necessary, using clippers rather than razors. | ||||
| Treat infections remote to the surgical site before elective surgery. | ||||
| Adequately control glucose levels preoperatively. | ||||
| Administer preoperative prophylactic antibiotics directed at the most common pathogens. | ||||
| To prevent Clostridium difficile infections: | B | 10, 13, 46 | ||
| Use routine contact precautions for patients with C. difficile infection or colonization. | ||||
| Minimize the frequency and duration of antibiotic therapy, and the number of antibiotic agents prescribed. | ||||
| Implement a systematic approach to reduce inappropriate antibiotic prescribing, such as an antibiotic stewardship program. | ||||
| To prevent infection with MRSA or other multidrug-resistant organisms: | B | 11, 12, 22, 52–54 | ||
| Use routine contact precautions for patients colonized or infected with multidrug-resistant organisms. | ||||
| Bathe patients in intensive care units daily with chlorhexidine. | ||||
| Administer decolonization therapy with mupirocin (Bactroban) to colonized patients if infection rates do not decrease despite basic prevention measures. | ||||
| Perform active surveillance cultures for MRSA and multidrug-resistant organisms if infection rates do not decrease despite basic prevention measures. | ||||
| Implement a systematic approach to reduce inappropriate antibiotic prescribing. | ||||
| Type of infection | Estimated number per year | Estimated cost per infection | Most common microbiologic etiologies |
|---|---|---|---|
| Surgical site infection | 157,500 | $12,000 to $35,000 | Staphylococcus aureus, coagulase-negative staphylococci, Escherichia coli |
| Catheter-associated urinary tract infection | 93,300 | $1,000 | E. coli, Pseudomonas aeruginosa, Klebsiella species |
| Clostridium difficile infection | 80,400 | $6,000 to $9,000 | C. difficile |
| Central line–associated bloodstream infection | 71,900 | $7,000 to $29,000 | S. aureus, coagulase-negative staphylococci, Enterococcus species |
| Ventilator-associated pneumonia | 49,900 | $20,000 to $29,000 | S. aureus, P. aeruginosa, Klebsiella species |
Most health care–associated infections can be prevented by risk factor modification, which is summarized in evidence-based guidelines.5–13 Preventing infections requires administrative support for implementing standardized practices on the part of the physician and regional and national health systems.14 A model regional initiative is the Michigan Keystone collaborative, which reduced central line–associated infections in intensive care units by two-thirds.15
General Recommendations
General recommendations for prevention of health care–associated infections include educating health care personnel about hand hygiene before and after any procedure or patient contact. Device-related infections can be reduced by inserting devices only when necessary, using sterile techniques, and removing the devices when they are no longer needed. Implementation of a group of evidence-based interventions, known as a bundle, has achieved greater reductions in rates of infections compared with one intervention implemented alone.16 A checklist is an important tool that physicians and hospitals can use to ensure adherence for each bundle component.15 Over the past decade, downward trends in most health care–associated infections have been reported nationwide.17,18
Central Line–Associated Bloodstream Infection
Central venous catheters include temporary central catheters, peripherally inserted central catheters, dialysis catheters, and more permanent tunneled catheters. Most central line–associated bloodstream infections are caused by microorganisms that colonize the skin, then spread from the catheter insertion site to the surface of the catheter, or through contamination of the catheter hub via hands or fomites.5
Indications for insertion of a central venous catheter include administration of medications that could cause harm if given through a peripheral vein, the need to provide large volumes of blood products or fluids, or the inability to obtain a peripheral vein. A peripherally inserted central catheter can be used for long-term intravenous therapy, but should not be inserted solely for reasons of patient comfort (e.g., reducing needlesticks for routine blood tests). For temporary catheters, the subclavian vein is the preferred insertion site to minimize the risk of infection; the femoral vein should be avoided, if possible.5,19 [ corrected]
The catheter should be inserted while the clinician is wearing a sterile gown, cap, mask, and gloves; a full-length sterile drape should be placed over the patient. The risk of central line–associated bloodstream infection is increased up to sixfold when these precautions are not used.20 For skin preparation, chlorhexidine reduces catheter bacterial colonization by 50% compared with povidone-iodine.21 Application of chlorhexidine dressings at the insertion site for all patients and daily chlorhexidine bathing for patients in intensive care units also reduce rates of central line–associated bloodstream infection.22-24 Additional practices to reduce infection include weekly replacement of transparent dressings and replacement of tubing every four to seven days. Daily reassessment can help ensure prompt removal of nonessential catheters.5
Catheter-Associated UTI
An estimated 15% to 25% of patients receive indwelling urinary catheters at some period during hospitalization. Although mortality associated with urinary tract infections (UTIs) is not as high as with other health care–associated infections, catheter-associated UTIs are the most common device-related health care–associated infection.1
Preventing catheter-associated UTIs begins with avoiding unnecessary catheterization. Up to 38% of catheter insertions are inappropriate.25 Examples of inappropriate use include substituting catheterization for nursing care in patients with incontinence, or for obtaining urine for diagnostic tests when the patient can voluntarily void.6 The duration of catheterization is also a risk factor; catheters that are no longer necessary should be removed promptly. The use of reminders or stop orders to prompt removal of unnecessary catheters has been demonstrated to reduce rates of catheter-associated UTIs by 53%.26 Catheters should be inserted only after performing hand hygiene, using aseptic technique and sterile equipment. Strong recommendations for maintenance involve maintaining a closed, unobstructed drainage system below the level of the bladder and routine cleaning of the meatal surface. Changing catheters at regular intervals, using topical antiseptics at the periurethral site, irrigating the bladder, and administering systemic antibiotics are not recommended.6
Ventilator-Associated Pneumonia
Ventilator-associated pneumonia can develop while the patient is being mechanically ventilated. It accounts for an estimated one-fifth of all pneumonias acquired during hospitalization,27 and occurs in an estimated 8% to 28% of mechanically ventilated patients.28 Mortality rates are estimated to be 30% to 70%.8 Identifying patients who may be candidates for noninvasive positive pressure ventilation is the first step in reducing rates of intubation.29 Additionally, avoiding reintubation reduces the risk of ventilator-associated pneumonia.
Respiratory equipment must be maintained, sterilized, and disinfected in accordance with evidence-based standards.30 Keeping the head of the bed in a semirecumbent position and performing antiseptic oral care, usually with chlorhexidine, reduce rates of ventilator-associated pneumonia.31,32 Although histamine H2 receptor blockers and proton pump inhibitors are commonly prescribed for patients who are mechanically ventilated and who are at high risk of stress ulcers, these drugs are associated with higher rates of ventilator-associated pneumonia and are not routinely recommended.7,33
Surgical Site Infection
Surgical site infections account for 24% of health care–associated infections, making them one of the most prevalent types.1 Most surgical site infections originate during the surgical procedure, mainly from endogenous skin or fecal flora. The risk of developing a surgical site infection varies depending on the type of surgery, patient age and comorbidities, timing of prophylactic antibiotics, and surgical technique.
Key preoperative factors that reduce the risk of subsequent infections include ensuring that any existing infections have resolved, and removing hair, if necessary, with clippers rather than razors.9,34,35 Controlling blood glucose levels before surgery is recommended, because the risk of infection is increased in patients with poorly controlled diabetes mellitus. One study demonstrated that having a preoperative A1C level less than 7% was associated with an odds reduction of 2.1 for surgical site infections.36 Guidelines for preoperative antibiotics recommend prophylaxis, typically within one hour of surgery, targeted at organisms likely to cause infections (usually cefazolin or a second-generation cephalosporin, or clindamycin or vancomycin for patients with a beta-lactam allergy).37,38 The most recent guidelines from the Centers for Disease Control and Prevention (CDC) state that chlorhexidine and povidone-iodine are appropriate for patient skin preparation; however, a randomized trial of 849 patients found that chlorhexidine resulted in a 40% decrease in rates of surgical site infection compared with povidone-iodine.39
Clostridium difficile Infection
Although rates of device- and procedure-related health care–associated infections have declined over the past decade, mortality from C. difficile infections increased nearly fivefold from 1999 to 2007, largely because of the emergence of the virulent BI/NAP1/027 strain.40 An estimated 94% of infections are acquired in the health care setting.41 About 14,000 deaths occur annually in the United States, and some data suggest that C. difficile has overtaken methicillin-resistant Staphylococcus aureus (MRSA) as the most common health care–associated microbial pathogen in some regions.40,42
Prevention efforts in acute care settings are focused on ensuring that the environment and equipment are disinfected with sporicidal disinfectants (e.g., bleach), and that infected patients are isolated. For clinicians, adherence to contact precautions (e.g., hand hygiene, gloves, gowns) is key for prevention of C. difficile transmission. Because there may be a delay of up to several months between acquisition of C. difficile and the presence of clinical manifestations—only one-fourth of infections manifest in the inpatient setting—the CDC recommends that patients be tested for C. difficile infection if they have diarrhea while receiving antibiotics or within several months of therapy.10,41
Exposure to antibiotics increases the risk of developing C. difficile infection; therefore, physicians must weigh the risks of antibiotic use vs. the benefits. Up to 50% of antibiotic use is inappropriate,43-45 including unnecessary prescribing and failure to discontinue therapy after a sufficient period. Therefore, physicians should limit the dosing frequency, duration of therapy, and number of antibiotics used to the minimum necessary to adequately treat the patient. Strategies to optimize antibiotic prescribing include adherence to standardized physician orders with preselected antibiotics based on clinical evidence, conversion of intravenous to oral formulations when possible, and de-escalation of broad empiric therapy once culture results are known.44,46 Two separate meta-analyses suggested that use of probiotics (most studies used Lactobacillus species) in hospitalized patients receiving antibiotics is associated with reduced rates of C. difficile infection.47,48
Multidrug-Resistant Organisms
Multidrug-resistant organisms are resistant to one or more classes of antibiotics, typically those that are most commonly used. Up to 16% of health care–associated infections are caused by multidrug-resistant organisms,49 primarily MRSA. Although MRSA is endemic in the United States, its prevalence in health care settings seems to have leveled off.3 Gram-negative organisms that exhibit resistance to extended-spectrum beta lactams or carbapenems are less common than MRSA, but are increasing in prevalence. Over a 10-year period, carbapenem-resistant Enterobacteriaceae increased 3.5-fold to 4.2% of all Enterobacteriaceae species.50 Few antibiotics are available to treat these infections, which have high rates of morbidity and mortality. Patients infected with carbapenem-resistant Klebsiella pneumoniae were 4.5 times as likely to die from their infection compared with those who had a susceptible strain.51
Strong recommendations for prevention include education of health care professionals, compliance with hand hygiene, and contact precautions for colonized or infected patients.12 For patients in intensive care units, daily bathing with chlorhexidine reduces rates of infection with multidrug-resistant organisms.22 In epidemic settings, performing active surveillance by screening high-risk patients for MRSA colonization and initiating decolonization therapy with mupirocin (Bactroban) in colonized patients have been shown to reduce MRSA infections.11,12,52 As with C. difficile infection, judicious use of antibiotics may reduce the prevalence of multidrug-resistant organisms in health care facilities.53,54
Data Sources: A PubMed search was completed in Clinical Queries as well as with the full database using the key terms prevention and infection, plus each of the following terms: central line, catheter-associated urinary tract, and surgical site. Additional search terms included prevention plus ventilator-associated pneumonia, Clostridium difficile, MRSA hospital, and multidrug-resistant organisms. Additional searches were performed in the Cochrane Library and the National Guideline Clearing-house. Search date: May 13, 2014.
