This is a corrected version of the article that appeared in print.
Am Fam Physician. 2014;90(9):664-666
Key Points for Practice
• Vaccines should be administered before planned immunosuppression, with live vaccines given four weeks in advance and inactivated vaccines given two weeks in advance.
• Immunocompetent persons who live with an immunocompromised patient can safely receive inactivated vaccines.
• Varicella and zoster vaccines should not be administered to highly immunocompromised patients.
• Annual vaccination with inactivated influenza vaccine is recommended for immunocompromised patients six months and older, except those who are unlikely to respond.
From the AFP Editors
Vaccination of immunocompromised patients is important because impaired host defenses predispose patients to an increased risk of vaccine-preventable infections. These patients also have a greater risk of exposure to pathogens because of their frequent contact with medical environments. Primary care physicians who provide care for immunocompromised persons share responsibility with subspecialists for ensuring that appropriate vaccines are administered to these patients and for recommending appropriate vaccinations for other members of the household. Recommended vaccination schedules for immunocompetent children and adults are published annually by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention. However, these schedules do not address vaccinations for immunocompromised persons who are at greater risk of morbidity and mortality from vaccine-preventable infections. To address this gap, the Infectious Diseases Society of America (IDSA) recently published evidence-based recommendations for vaccinations in immunocompromised persons and their household members.
The guideline covers children and adults with primary (congenital) immunodeficiency; those with secondary immunodeficiency caused by human immunodeficiency virus (HIV) infection, cancer chemotherapy, stem cell or solid organ transplant, sickle cell disease, and surgical asplenia; and patients with chronic inflammatory diseases who are receiving systemic corticosteroids, immunomodulators, or biologic agents. The guideline includes several tables, one for each condition, that list specific vaccines that are recommended and contra-indicated, with the level of evidence associated with each recommendation. Some of the recommendations distinguish between high- and low-level immunosuppression. High-level immunosuppression includes patients who have a primary immunodeficiency; who are receiving chemotherapy; who have received a solid organ transplant within the previous two months; who have HIV infection and a CD4 cell count less than 200 per mm3 (0.20 × 109 per L; for adults and older children) or less than 15% (for infants and young children); who are receiving daily corticosteroid therapy equivalent to 20 mg of prednisone or greater for at least 14 days; or who are receiving biologic immunomodulators. After hematopoietic stem cell transplant, the duration of high-level immunosuppression depends on the type of transplant (longer for allogenic than for autologous); type of donor and stem cell source; and posttransplant complications, such as graft vs. host disease.
Planned Immunosuppression
When feasible, vaccines should be administered before planned immunosuppression. Live vaccines should be given at least four weeks in advance and should be avoided in the two weeks before immunosuppression is started. Inactivated vaccines should be administered at least two weeks in advance.
Vaccination in Household Members
Immunocompetent persons who live in the same household as the immunocompromised patient can safely receive inactivated vaccines according to the recommended schedule from ACIP. If the immunocompromised patient is six months or older, household members may receive the inactivated influenza vaccine, or the live attenuated influenza vaccine if they are healthy, not pregnant, and two to 49 years of age. Exceptions include those who live with an immunocompromised person who received a hematopoietic stem cell transplant in the previous two months, who has graft vs. host disease, or who has severe combined immunodeficiency. Live attentuated influenza vaccine should not be administered to these persons or, if administered, contact between the immunocompromised patient and household member should be avoided for seven days. [ corrected]
Healthy immunocompetent persons who live with an immunocompromised patient should receive the following live vaccines based on ACIP's recommended schedule: combined measles, mumps, and rubella (MMR); rotavirus for infants two to seven months of age; varicella; and zoster (Table 1). These persons can safely receive the yellow fever and oral typhoid vaccines for travel. Oral polio vaccine should not be administered to persons who live with an immunocompromised patient.
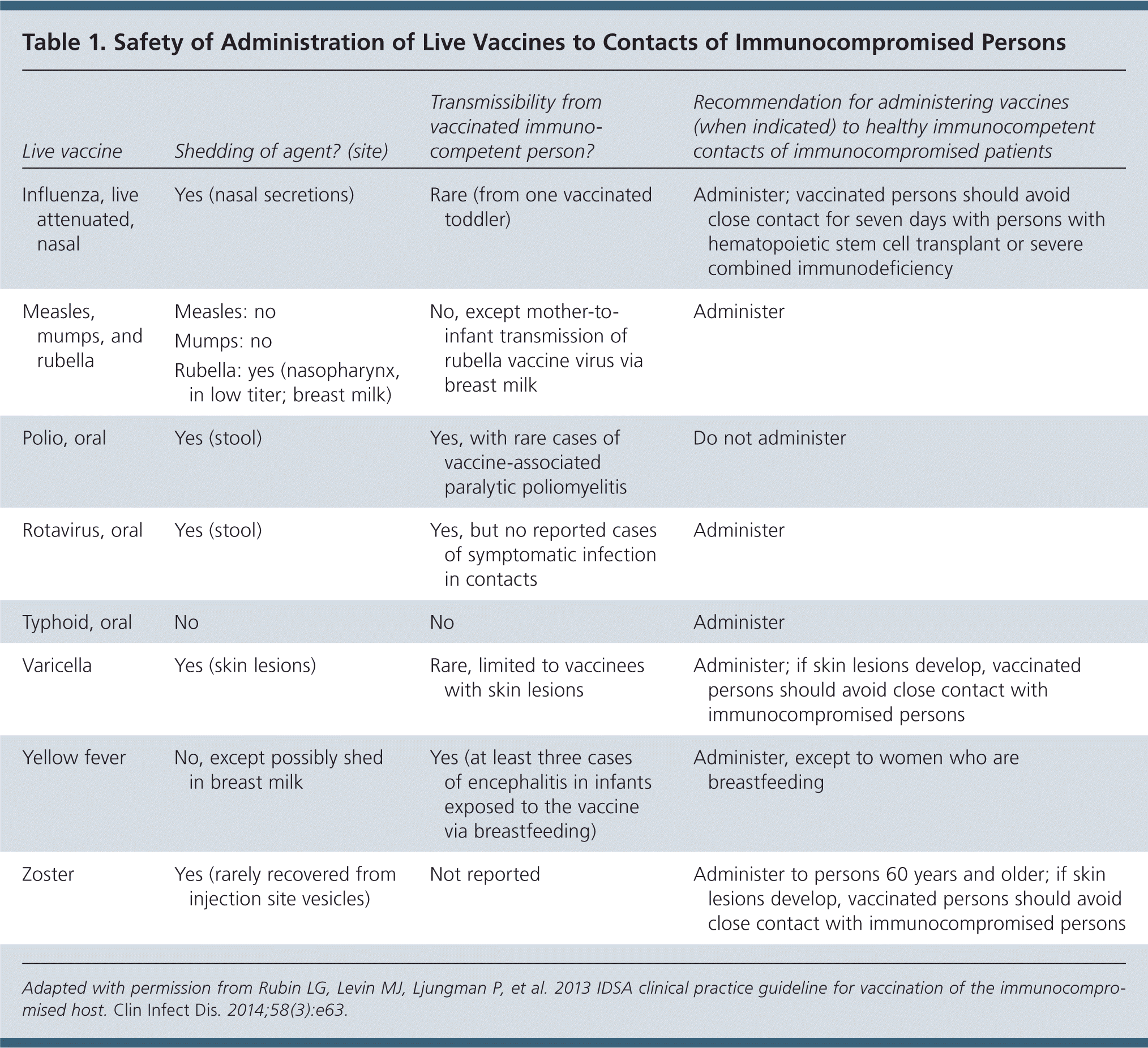
| Live vaccine | Shedding of agent? (site) | Transmissibility from vaccinated immunocompetent person? | Recommendation for administering vaccines (when indicated) to healthy immunocompetent contacts of immunocompromised patients |
|---|---|---|---|
| Influenza, live attenuated, nasal | Yes (nasal secretions) | Rare (from one vaccinated toddler) | Administer; vaccinated persons should avoid close contact for seven days with persons with hematopoietic stem cell transplant or severe combined immunodeficiency |
| Measles, mumps, and rubella | Measles: no | No, except mother-to-infant transmission of rubella vaccine virus via breast milk | Administer |
| Mumps: no | |||
| Rubella: yes (nasopharynx, in low titer; breast milk) | |||
| Polio, oral | Yes (stool) | Yes, with rare cases of vaccine-associated paralytic poliomyelitis | Do not administer |
| Rotavirus, oral | Yes (stool) | Yes, but no reported cases of symptomatic infection in contacts | Administer |
| Typhoid, oral | No | No | Administer |
| Varicella | Yes (skin lesions) | Rare, limited to vaccinees with skin lesions | Administer; if skin lesions develop, vaccinated persons should avoid close contact with immunocompromised persons |
| Yellow fever | No, except possibly shed in breast milk | Yes (at least three cases of encephalitis in infants exposed to the vaccine via breastfeeding) | Administer, except to women who are breastfeeding |
| Zoster | Yes (rarely recovered from injection site vesicles) | Not reported | Administer to persons 60 years and older; if skin lesions develop, vaccinated persons should avoid close contact with immunocompromised persons |
Highly immunocompromised patients should avoid handling diapers of infants who have received rotavirus vaccine for four weeks after vaccination. Immunocompromised patients should avoid contact with persons who develop skin lesions after receiving varicella or zoster vaccines until the lesions resolve.
Varicella and Zoster Vaccination
Varicella vaccine should not be administered to highly immunocompromised patients. However, select patients (e.g., those with HIV infection who are not highly immunocompromised, those with a primary immunodeficiency without defective T cell–mediated immunity) should receive two doses of vaccine three months apart. Varicella vaccination can be considered in patients who do not have evidence of immunity (i.e., age-appropriate varicella vaccination, serologic evidence of immunity, clinician-diagnosed or -verified history of varicella or zoster, or laboratory-proven varicella or zoster) and who are receiving long-term, low-dose immunosuppressant drugs. When indicated, varicella vaccine should be administered as a single-antigen product and not combined with the MMR vaccine.
Zoster vaccine should be administered to patients 60 years and older who are receiving therapy to induce low-level immunosuppression. The vaccine should not be administered to highly immunocompromised patients.
Influenza Vaccination
Annual administration of inactivated influenza vaccine is recommended for immunocompromised patients six months and older, except those who are unlikely to respond (e.g., those receiving intensive chemotherapy, those who have received anti–B-cell antibodies within the previous six months). Live attenuated influenza vaccine should not be administered to immunocompromised persons.
Guideline source: Infectious Diseases Society of America
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Published source: Clinical Infectious Diseases, February 1, 2014
Available at: http://cid.oxfordjournals.org/content/58/3/e44.full