Am Fam Physician. 2014;90(11):784-789
Related editorial: How Should We Manage Incidentalomas?.
Patient information: See related handout on incidentalomas, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Incidentalomas are increasingly common findings on radiologic studies, causing worry for physicians and patients. Physicians should consider the risk of discovering incidentalomas when contemplating imaging. Patients may assume that incidentalomas are cancer, and may not be aware of the radiation risks associated with repeat imaging. Once incidentalomas are detected, appropriate management is dependent on an informed patient's wishes and the clinical situation. Guidelines are provided for the initial management of eight incidentalomas (pituitary, thyroid, pulmonary, hepatic, pancreatic, adrenal, renal, and ovarian). Patients presenting with pituitary incidentalomas should undergo pituitary-specific magnetic resonance imaging if the lesion is 1 cm or larger, or if it abuts the optic chiasm. Thyroid incidentalomas are ubiquitous, but nodules larger than 1 to 2 cm are of greater concern. Worrisome pulmonary incidentalomas are those larger than 8 mm or those with irregular borders, eccentric calcifications, or low density. However, current guidelines recommend that even pulmonary incidentalomas as small as 4 mm be followed. Solid hepatic incidentalomas 5 mm or larger should be monitored closely, and multiphasic scanning is helpful. Pancreatic cystic neoplasms have malignant potential, and surgery is recommended for pancreatic cysts larger than 3 cm with suspicious features. Adrenal lesions larger than 4 cm are usually biopsied. The Bosniak classification is a well-accepted means of triaging renal incidentalomas. Lesions at category IIF or greater require serial monitoring or surgery. Benign or probably benign ovarian cysts 3 cm or smaller in premenopausal women or 1 cm or smaller in postmenopausal women do not require follow-up. Ovarian cysts with thickened walls or septa, or solid components with blood flow, should be managed closely.
Advanced imaging studies show tremendous details of pathology. They can also show incidental findings that are of unclear significance. Family physicians are often the ones to explain these incidentalomas to patients. Further workup of incidentalomas may cause harm. According to an expert panel of the American College of Radiology (ACR), although most incidental findings prove to be benign, their discovery often leads to a cascade of testing that is costly, provokes anxiety, exposes patients to radiation unnecessarily, and may even cause morbidity.1 The goal of this review is to provide guidelines on initial management of incidentalomas. Available guidelines are from specialty societies and based on expert opinion, and do not always agree.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| MRI with pituitary protocol is the preferred imaging modality to further characterize pituitary lesions. | C | 3 |
| Most thyroid nodules 1 cm or smaller can be followed with ultrasonography in six months. | C | 4, 5 |
| Pulmonary nodules 4 mm or smaller in low-risk patients do not warrant further imaging. | C | 11 |
| Multiphasic CT or MRI is preferred for characterizing many hepatic incidentalomas. | C | 1, 13 |
| Pancreatic cystic neoplasms of any size require monitoring because they have malignant potential. | C | 1 |
| In low-risk, asymptomatic patients, adrenal incidentalomas 4 cm or smaller are often benign. | C | 19–23 |
| The Bosniak classification should be used to manage renal incidentalomas. | C | 1, 26, 27 |
| Immediate ultrasonography should be performed to further assess benign-appearing ovarian cysts larger than 5 cm in early postmenopausal women or larger than 3 cm in late postmenopausal women. | C | 30 |
| When evaluating an incidentaloma, previous imaging results should be obtained, if available, to assess lesion stability. | C | 1, 11, 12 |
| When determining the need for further workup of incidentalomas, the overall health, life expectancy, and personal wishes of the patient should be considered, and the risk of harm from further imaging should be discussed. | C | 1, 11, 12 |
Pituitary
Pituitary incidentalomas are found in at least one in 10 persons and are clinically significant in approximately one in 1,000.2 Lesions 1 cm or larger with a solid component are more likely to grow and cause symptoms. Magnetic resonance imaging (MRI) with pituitary protocol is the preferred study to characterize lesions, even if computed tomography (CT) was performed initially.3 The differential diagnosis includes pituitary adenoma, Rathke cleft cyst, craniopharyngioma, and hyperplasia.
The Endocrine Society recommends a detailed visual field and eye examination for lesions 1 cm or greater or for those that abut or compress the optic nerve or chiasm, with consideration of surgery for any defects found.3 It recommends testing for hypersecretion of various pituitary hormones, but this is based on weak evidence. Assessment of prolactin levels is helpful because many prolactinomas can be managed medically. Most other functional and nonfunctional lesions should be monitored and/or resected. Repeat MRI is recommended in six to 12 months, depending on the size of the lesion. An algorithm adapted from these guidelines is available at https://www.aafp.org/afp/2013/0901/p319.html.
Thyroid
Thyroid lesions are often discovered on CT, MRI, neck and carotid ultrasonography, and positron emission tomography (PET). About one-half of patients undergoing imaging or autopsy have thyroid nodules.4 Spongiform cysts, which contain an aggregation of microcysts, and simple cysts are benign.5 Traditional estimates of malignancy for thyroid nodules exceed 5% (with a greater risk in PET-identified lesions), although a more recent estimate is less than 2%.6,7
Further history, including radiation exposure and family history; a physical examination to detect enlarged lymph nodes or hoarseness; targeted ultrasonography; and measurement of thyroid-stimulating hormone (TSH) levels are recommended after nodules are detected.5 In about 5% of patients, a low TSH level will reveal a hyperfunctioning benign nodule; a radionuclide scan should be done for confirmation.5 Nodules associated with high TSH levels have a greater risk of malignancy. An algorithm for the assessment of nodules associated with normal and high TSH levels is available at https://www.aafp.org/afp/2013/0801/p193.html.
Most nodules 1 cm or smaller can be followed with ultrasonography in six months.4,5 Fine-needle aspiration biopsy should be considered for smaller nodules in patients with risk factors (e.g., history of radiation of the neck in childhood, family history of thyroid cancer, isolated uptake on PET scanning, suspicious cervical lymphadenopathy, hoarseness).5 Fine-needle aspiration is recommended for nodules larger than 1 to 2 cm with hypoechogenicity, microcalcifications, or intranodular vascularity.5 Experts from the University of California, San Francisco, have questioned the 1-cm cutoff for biopsy, based on a study in which more than 98% of nodules in a population of 8,800 patients proved to be benign.7 They recommend biopsy only for solid nodules with microcalcifications and for those larger than 2 cm.
Pulmonary
Any new pulmonary nodule in a patient with a history of lung cancer is ominous, and the risk of malignancy approaches 25% in those with a history of extrathoracic malignancy.8 However, many nodules are incidentally discovered in low-risk patients. Findings of incidental solitary pulmonary nodules are common in CT studies, with a prevalence of 8% to 51%.9 Risk factors include age, smoking history, asbestos exposure, family history, and a history of malignancy. Worrisome nodules include those that are larger than 8 mm or that have irregular borders, eccentric calcifications (or lack of calcification), or low density.9,10 Some lesions with a benign calcification pattern (e.g., old granulomas) or a clear vascular pattern of hamartoma or arteriovenous malformation can be considered benign.
The 2013 American College of Chest Physicians (ACCP) guidelines for management of pulmonary nodules state that nodules 4 mm or smaller in a low-risk patient do not warrant further imaging.11 All other scenarios involve repeat CT over six to 24 months, except in high-risk patients, who should be reevaluated in three months. The ACCP recommends the use of low-dose, non–contrast-enhanced CT to minimize radiation exposure. Ground-glass or subsolid nodules are suspicious for early adenocarcinoma.11 For these, the cutoff for further surveillance is at least 6 mm and involves repeat CT (with thin sections) annually for at least three years. The ACCP has a low threshold for recommending PET scans and biopsy; PET scans are recommended for nodules 8 mm or larger in low- to moderate-risk patients with pretest probability of malignancy of 5% to 65%.11 The ACCP endorses the risk calculator available at http://www.nucmed.com/nucmed/SPN_Risk_Calculator.aspx.
To mitigate patient fear about monitoring pulmonary nodules, physicians should use lay terms, show the CT image, calculate the risk of cancer, and avoid the use of medical jargon or dismissive language.12
Hepatic
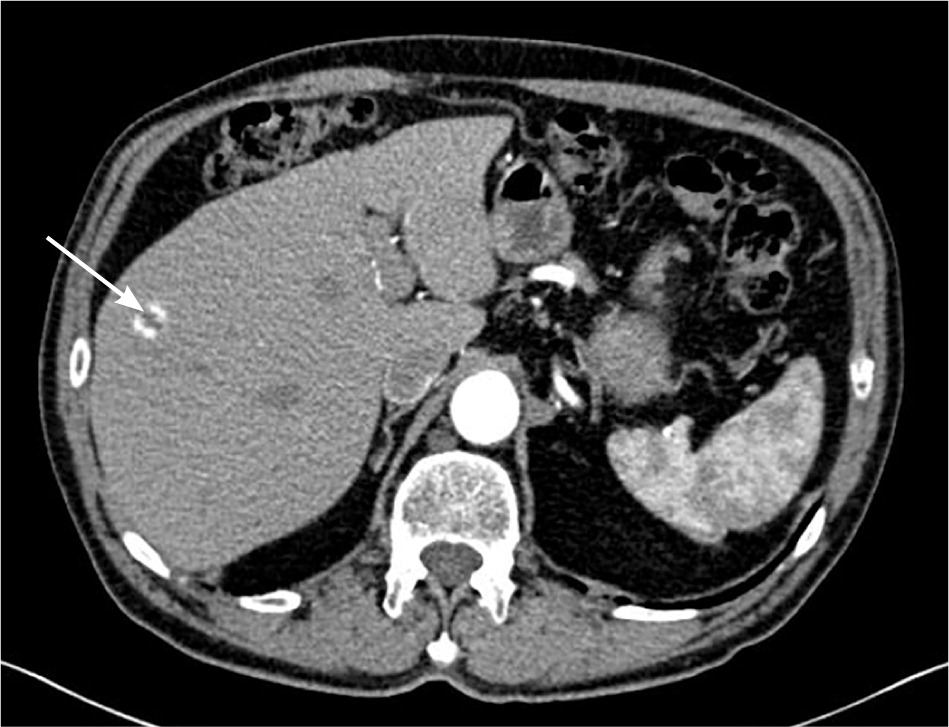
The ACR recommends no further workup in low-risk patients who have lesions smaller than 5 mm, or larger lesions with low attenuation (20 Hounsfield units [HU] or less) or benign imaging features.1 Lesions of any size with “flash-filling” (robust enhancement) are usually benign hemangiomas. Suspicious findings include solid lesions 5 mm or larger, or those with ill-defined margins, enhancement greater than 20 HU, a heterogenous appearance, or interval growth.1,13
Simple liver cysts may not require further evaluation or monitoring. Indeterminate primarily cystic lesions may need to be followed by ultrasonography until they have been confirmed benign. Benign cystadenomas are rare and seldom undergo malignant transformation; however, further workup may be warranted because imaging cannot reliably distinguish between benign and malignant cystadenomas.14 Cavernous hemangiomas, which show peripheral nodular enhancement on CT, account for most benign liver incidentalomas. Risk of rupture is exceedingly low in those smaller than 11 cm, and they are not treated in asymptomatic patients.13
Hepatic adenomas are found in women of reproductive age and are often associated with oral contraceptive use.13 Oral contraceptives should be discontinued, and serial imaging should be performed. Pregnancy exacerbates lesions; therefore, nonhormonal contraception should be used. Larger or persistent lesions carry some risk of malignant transformation and bleeding, and surgical resection should be considered. Focal nodular hyperplasia describes benign tumors that also occur in young women. Unlike hemangiomas, their arterial feed is centrally located and may be described as a central scar on imaging.
Standard abdominal CT is a single-phase study that is unable to adequately characterize hepatic lesions. Multiphasic CT or MRI is preferred.1,13 Percutaneous biopsy is sometimes required and carries a low complication rate of 2.0% to 4.8%.1 Surgical resection is warranted in patients for whom hepatocellular carcinoma cannot be excluded.
Pancreatic
Pancreatic cysts are found in more than 2% of patients undergoing abdominal CT or MRI15 (Figure 2). Classification of cysts can be challenging, and pancreatic cystic neoplasms may have malignant potential.16 Serous cystic tumors are rarely malignant, and are resected only if growth or symptoms occur.1 The other three subtypes—mucinous cystic neoplasms, intraductal papillary mucinous neoplasms, and solid pseudopapillary neoplasms—are potentially malignant. In a retrospective study of 78 patients with pancreatic cystic neoplasms, fewer than 5% of lesions smaller than 3 cm were malignant compared with more than 15% of larger lesions.17
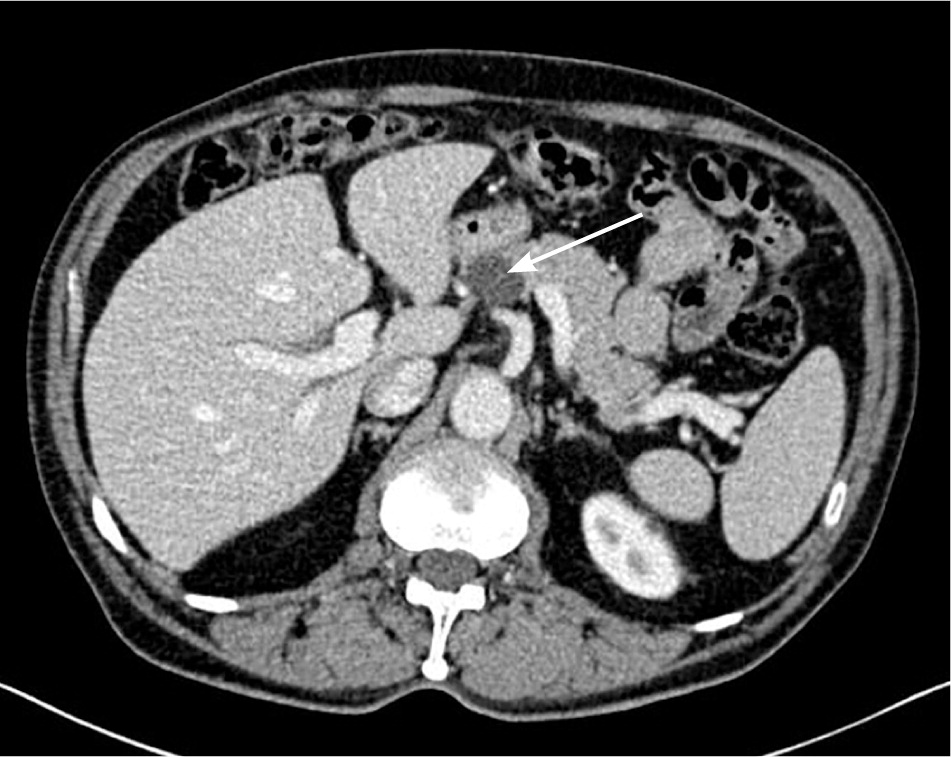
Cystic lesions without malignant potential include pseudocysts and non-neoplastic pancreatic cysts. Pseu-docysts are common, lack epithelial lining, and often occur after pancreatitis. Non-neoplastic pancreatic cysts (e.g., true cysts, retention cysts, mucinous non-neoplastic cysts, lymphoepithelial cysts) are rare.
The ACR recommends MRI to further characterize pancreatic cysts.1 Surgery is recommended for cysts larger than 3 cm with suspicious features (e.g., mural nodules, lymphadenopathy, involvement of bile or pancreatic ducts). Aspiration is recommended for smaller cysts if surgery is being considered; otherwise, radiologic monitoring is suggested for lesions 1 to 3 cm in size. The American College of Gastroenterology recommends endoscopic ultrasonography with aspiration of cysts for biochemical marker analysis to stratify risk.18
Adrenal
Adrenal lesions are detected on 3% to 4% of CT and MRI studies.19 One study showed that 1.2% of lesions are malignant, and all exceeded 5 cm.20 Metastatic lesions are rare in patients without a history of cancer and, when present, are associated with bronchogenic carcinoma, renal cell carcinoma, and melanoma.21
Lesions smaller than 1 cm with fatty or cyst-like consistency do not require further workup. Lesions larger than 4 cm should be further evaluated with special imaging modalities and are usually biopsied unless they are convincingly benign, such as cysts or myelolipomas. Lesions 1 to 4 cm in size are often benign adenomas.21
A benign lesion can be confirmed in one of three ways: stability over six to 12 months, low attenuation (10 HU or less on unenhanced CT), or confirmation of lipid-rich adenoma through chemical-shift MRI.1,21,22 A subset of benign lesions may be lipid-poor or have HU greater than 10. For these indeterminate lesions, an adrenal CT protocol includes CT washout tests. Malignant lesions have a low washout.22
In patients with no history or physical findings to suggest hyperfunctioning lesions, physicians may defer a hormonal workup, although endocrinologists from the Mayo Clinic recommend annual monitoring.1,23 Such monitoring includes 24-hour urine cortisol and metanephrine/catecholamine levels, and plasma aldosterone and renin testing.
In 2012, the ACR updated their appropriateness criteria for management of adrenal incidentalomas.24
Renal
The incidence of renal cysts increases with age. They are discovered in up to one-third of older adults25 (Figure 3). Almost all simple renal cysts are benign, and patients can be reassured; however, any complex cyst or mass requires further evaluation.26 With complex cysts, the most important feature to determine malignant potential is enhancement with contrast media. However, enhancement can be equivocal or more noticeable on MRI compared with CT. Consultation with a radiologist is helpful in these cases.
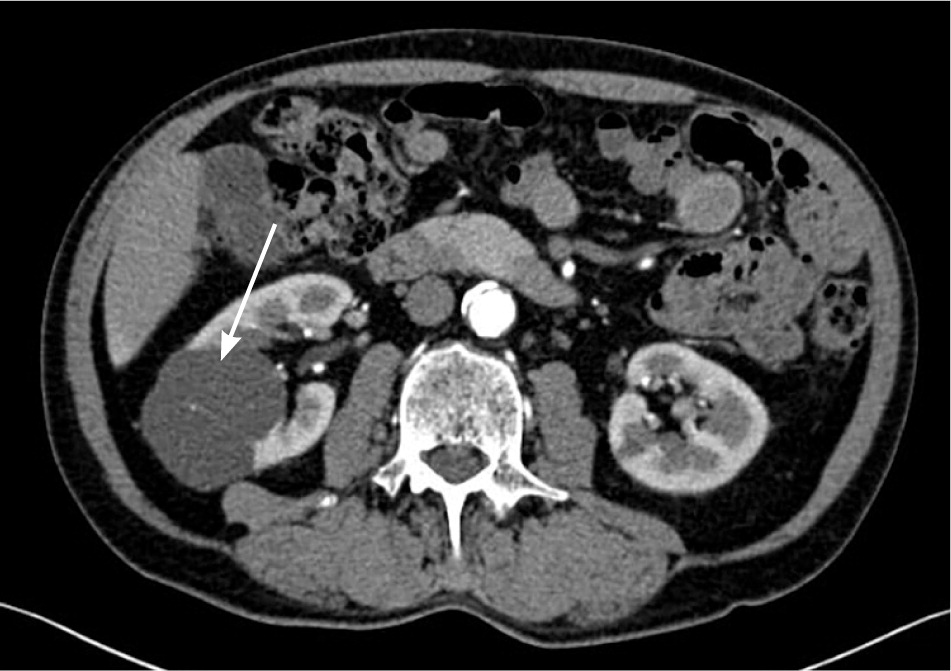
The Bosniak classification is a well-accepted method to triage renal lesions.1,27 Lesions classified as category I (benign simple cyst) or II (benign cystic lesion with some complex features) do not need to be followed. Nonenhancing complex cysts larger than 3 cm (category IIF) have a 5% to 10% risk of malignancy and should be followed with imaging, although the frequency of monitoring is not well defined. Malignancy risk approaches 50% in similar lesions that are more complicated and that do enhance (category III). Category IV lesions include more clearly malignant cystic masses. Suspicious complex renal cysts or masses are usually not biopsied because of the risk of sampling error (exceptions are suspected abscess, lymphoma, or metastatic lesions). Surgical resection is the rule; however, surgery may be avoided in the case of fat-containing solid lesions with the appearance of benign angiomyolipomas.26
Ovarian
According to the 2007 consensus guidelines of the American College of Obstetricians and Gynecologists, most ovarian incidentalomas are benign and are often functional cysts in premenopausal women and cystadenomas in postmenopausal women.28 Risk of malignancy is much greater in postmenopausal women with complex cysts and masses. Transvaginal ultrasonography is the preferred imaging modality to further characterize these incidentalomas.
The Society of Radiologists in Ultrasound has published consensus guidelines showing images of low- and high-risk cysts, with recommendations for management.29 Physiologic cysts 3 cm or smaller in premenopausal women and simple cysts 1 cm or smaller in postmenopausal women are considered normal. For other lesions, frequency of monitoring is not well defined but may range from six to 12 weeks for more concerning lesions, or even yearly for lesions such as dermoids or endometriomas.
The ACR guidelines on adnexal incidentalomas are more stringent.30 Rather than simple cysts, the ACR classifies lesions as benign-appearing cysts (BACs; oval or round, unilocular, and regular/smooth wall, including hemorrhagic cysts) or probably benign cysts (PBCs; similar to benign-appearing cysts but with angulated margins, not round or oval, or not adequately imaged). Immediate ultrasonography should be performed in the following patient groups to further assess ovarian incidentalomas: premenopausal women with PBCs larger than 5 cm; early postmenopausal women with BACs larger than 5 cm or PBCs larger than 3 cm; and late postmenopausal women with BACs larger than 3 cm or PBCs larger than 1 cm. Pre-menopausal women with BACs larger than 5 cm or PBCs 3 to 5 cm should have repeat ultrasonography in six to 12 weeks, and early postmenopausal women with BACs 3 to 5 cm should have repeat ultrasonography in six to 12 months.
In most cases, fibromas, benign calcifications without associated masses, hydrosalpinx, and peritoneal inclusion cysts need not be followed. Endometriomas and cystic teratomas have very low malignant transformation but should be followed with ultrasonography. Red flag characteristics for adnexal lesions include thickened walls/septa and solid components with blood flow.28–30
Elevated levels of cancer antigen 125 have low sensitivity and specificity in premenopausal women but may indicate malignancy in postmenopausal women with ovarian incidentalomas.28
Final Comment
The best way to prevent incidentalomas is to avoid unnecessary imaging. When found, incidentalomas are rarely cause for alarm, but Table 1 lists red flag features to help determine when additional evaluation is warranted. Previous imaging studies should be obtained; these may show stability, which can obviate the need for further workup.1,11,12 The overall health, life expectancy, and wishes of the patient should be considered.

| Incidentaloma type | Red flags |
|---|---|
| Pituitary | Optic nerve impingement or vision changes |
| Signs and symptoms of hormonal hyper- or hyposecretion | |
| Size ≥ 1 cm with a solid component | |
| Thyroid | Elevated thyroid-stimulating hormone level |
| History of neck radiation or family history of thyroid malignancy | |
| Isolated uptake on positron emission tomography | |
| Solid nodule > 1 to 2 cm | |
| Suspicious cervical lymphadenopathy or hoarseness | |
| Pulmonary | History of extrathoracic malignancy |
| History of smoking or asbestos exposure | |
| Irregular borders, eccentric calcifications or lack of calcifications, low density | |
| Solid nodule > 8 mm | |
| Hepatic | Ill-defined margins, enhancement > 20 Hounsfield units, heterogeneous appearance, interval growth |
| Solid lesion ≥ 5 mm | |
| Suspected extrahepatic malignancy | |
| Underlying liver disease | |
| Pancreatic | Any cyst (not pseudocyst) > 3 cm |
| Pancreatic cystic neoplasms with malignant potential | |
| Adrenal | High attenuation on computed tomography (> 10 Hounsfield units) |
| Lipid-poor lesions | |
| Solid lesion > 4 cm | |
| Renal | Bosniak category IIF or greater |
| Complex cyst (multiple septations) > 3 cm | |
| Enhancement with contrast media | |
| Wall thickening or calcification | |
| Ovarian | Elevated cancer antigen 125 level in postmenopausal women |
| Size > 3 cm in premenopausal women or > 1 cm in postmenopausal women | |
| Thickened wall/septa, solid components with blood flow |
Radiation risks should be discussed because CT can be equivalent to more than 100 plain radiography films of the chest. Radiation doses can be found at http://www.radiologyinfo.org/en/safety/index.cfm?pg=sfty_xray. A discussion with the radiologist may help optimize the study ordered or reveal an alternative with lower radiation exposure. Physicians should engage in shared decision making with the patient—with emphasis on the patient's values—while ensuring that he or she understands the risks of further imaging and interventions.
Data Sources: We searched Essential Evidence Plus, the Cochrane Database of Systematic Reviews, Clinical Evidence, the National Guideline Clearinghouse, the Agency for Healthcare Research and Quality evidence reports, and PubMed using the keywords incidentaloma, pituitary, thyroid, pulmonary, hepatic, pancreatic, adrenal, renal, and ovarian. The search included observational studies, case reports, reviews, and consensus guidelines. Search dates: April 24, 2013, through August 8, 2014.