Am Fam Physician. 2014;90(12):863-866
Author disclosure: No relevant financial affiliations.
Lorcaserin (Belviq) is a selective serotonin 2C agonist labeled for chronic weight management in adults who are obese (body mass index [BMI] ≥ 30 kg per m2) or who are overweight (BMI ≥ 27 kg per m2) with at least one weight-related comorbidity. Similar to other weight-loss medications, lorcaserin should be used in conjunction with diet and exercise.
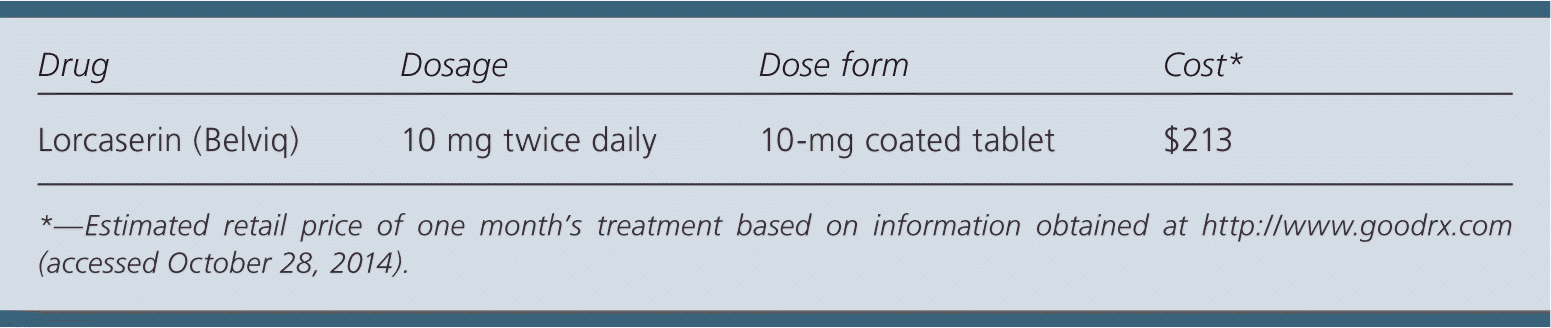
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Lorcaserin (Belviq) | 10 mg twice daily | 10-mg coated tablet | $213 |
SAFETY
Initial studies demonstrate that lorcaserin is safe when taken at the recommended dosage of 10 mg twice daily. Because serotonin agonists such as lorcaserin can cause serotonin syndrome, the manufacturer recommends that patients taking other serotonergic drugs such as antidepressants avoid coadministration of lorcaserin. Valvulopathy, a risk with previous weight-loss drugs, has not been demonstrated with lorcaserin; however, the medication has not been evaluated in patients with significant valvulopathy or congestive heart failure. Its long-term effects are unclear because it has not been studied for a period longer than two years.
Psychiatric symptoms including euphoria, hallucination, and dissociation rarely occur with recommended dosages, but these have occurred in 19% of patients taking 40 to 60 mg daily.1 Physical dependence has not been observed with lorcaserin, although psychic dependence is possible with higher dosages used to induce euphoria. Lorcaserin does not increase suicidal thoughts or actions, and does not cause mood changes or trouble sleeping when used as recommended.
Prolactin levels may be increased in some patients taking lorcaserin, although galactorrhea and gynecomastia were not reported in a premarketing study of 6,888 patients.1 Hypoglycemia is more common in patients with type 2 diabetes mellitus. Lorcaserin is a pregnancy category X drug because of the risk of fetal harm from weight loss and the risk of hypoglycemia.
TOLERABILITY
Lorcaserin is well tolerated. In early studies, less than 1% of patients discontinued treatment because of dizziness, and about 2% discontinued because of headache.2 In premarketing studies, more patients discontinued lorcaserin than placebo because of adverse effects, but overall dropout rates were similar.1
EFFECTIVENESS
Lorcaserin, used in combination with diet and exercise, results in modest weight loss of about 12.9 lb (5.8 kg) compared with 5.6 lb (2.5 kg) with placebo.3 On average, about 47% of patients will lose at least 5% of their body weight (about 11.1 lb [5 kg]) compared with 23% of patients receiving placebo (number needed to treat [NNT] = 4).1–3 About 22% of patients will lose at least 10% of their body weight vs. 9% of patients receiving placebo (NNT = 7). This weight loss is maintained, on average, for two years. Weight loss occurs early, and those who do not lose 5% of their body weight in the first three months are unlikely to achieve 5% loss by 52 weeks. Weight loss also occurs in patients with type 2 diabetes who are not taking insulin, although not to the same extent.4 Stopping lorcaserin after one year results in an average weight gain that is within 2.2 lb (1 kg) of baseline.1–3 There is no research on the effect of lorcaserin on mortality, incidence of diabetes, or other patient-oriented outcomes.
PRICE
Lorcaserin costs approximately $213 for a 30-day supply. In comparison, phentermine/topiramate (Qysmia), another prescription weight-loss medication, costs $160 for a 30-day supply. Compared with nonpharmacologic interventions, lorcaserin is much more expensive.
SIMPLICITY
The recommended dosage is 10 mg twice daily. The manufacturer recommends 12 weeks of therapy to determine its effectiveness.
Bottom Line
Lorcaserin, combined with diet and exercise, can be used to produce additional weight loss, although the effect on morbidity and mortality is not known. It should be discontinued in patients who do not lose at least 5% of their body weight in the first 12 weeks because they are unlikely to have any additional weight loss. Initial two-year studies did not demonstrate cardiac valvulopathy, but these studies excluded patients with significant cardiac disease. Euphoric effects at supratherapeutic dosages make the potential for recreational drug abuse a valid concern.
Editor's Note: On February 13, 2020, the U.S. Food and Drug Administration (FDA) requested that the manufacturer of Belviq (lorcaserin) voluntarily withdraw this drug from the market because a safety clinical trial showed an increased occurrence of cancer that outweighed any potential weight loss benefits. See the FDA Drug Safety Communication.