
Am Fam Physician. 2015;91(1):38-44
Author disclosure: No relevant financial affiliations.
Diagnosis of neuromuscular disorders in young children is often delayed for years after symptoms emerge, resulting in missed opportunities for therapy and genetic counseling. Identification of the weak child begins with careful attention to caregiver concerns and developmental surveillance at well-child visits. Family and medical histories can differentiate inherited from acquired causes of weakness. Physical examination should include observation of age-appropriate motor skills such as pull-to-sit, sitting, rising to stand, and walking/running. Serum creatine kinase levels should always be measured in children exhibiting neuromuscular weakness. Referrals to early intervention programs should not be postponed pending definitive diagnosis. If motor delay does not improve with early intervention, referral to a pediatric neurologist for diagnostic assessment is recommended. Tongue fasciculations, loss of motor milestones, or creatine kinase level greater than three times the normal limit should prompt immediate neurology referral. Once a neuromuscular disorder is diagnosed, the primary care clinician can help the family navigate subspecialty visits and consultations, advocate for services in the school and home, and help them cope with the emotional stresses of caring for a child with special needs.
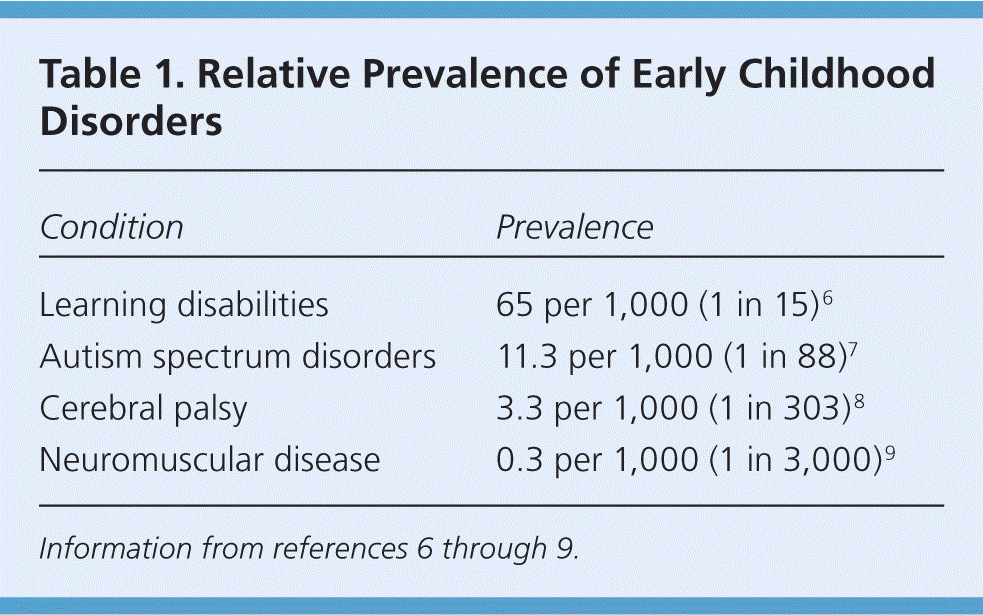
The diagnosis of neuromuscular disorders, commonly defined as acquired and inherited conditions of the muscles, nerves, and neuromuscular junction,1 is often delayed. For example, the average time from first parental concerns to diagnosis of Duchenne muscular dystrophy is more than two years.2,3 Only 10% of children with developmental delay receive services for which they are eligible by two years of age.4 Although caregiver characteristics and other socioeconomic factors affect timing of access to special services, medical practice also plays a role. Because neuromuscular disorders are rare, accounting for only a fraction of the approximately 9.6 million children in the United States with developmental disabilities5 (Table 16–9 ), physician experience may be inadequate to ensure timely identification.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Surveillance for developmental delay (including gross and fine motor function) should be performed at all well-child visits. | C | 6, 23, 32, 33 |
| When the evaluation for developmental delay suggests a peripheral neuromuscular problem, serum creatine kinase levels should be measured. | C | 23, 38 |
| Referral to early intervention programs should be made as soon as developmental delay is identified in an infant or toddler. | C | 23, 43 |
Importance of Early Diagnosis
Neuromuscular disorders place a financial and emotional burden on patients, families, and caregivers, as well as their communities.10–14 Early identification and diagnosis can help relieve caregiver stress and ensure appropriate management and services.15 In some cases, initiation of treatment can slow disease progression and improve outcomes (e.g., corticosteroids for Duchenne muscular dystrophy,16 enzyme replacement therapy for Pompe disease,17 medications for congenital myasthenic syndrome1). Genetic counseling enables informed decision making for future pregnancies. Recognition and accurate diagnosis also facilitate participation in treatment trials.
Screening (administration of a brief standardized tool aiding in the identification of children at risk of a developmental disorder) and surveillance (a flexible, longitudinal, continuous, and cumulative process whereby knowledgeable health care professionals identify children who may have developmental problems) of early childhood development are key components in the care of children.18,19 Not all clinicians adhere to standardized guidelines for developmental assessment, such as Bright Futures (http://brightfutures.aap.org).20,21 Basing assessments on clinical judgment alone can miss developmental delay in two-thirds of patients.22
To promote earlier recognition of neuromuscular diseases in children, the National Task Force for the Early Identification of Childhood Neuromuscular Disorders, a program partially funded by the Centers for Disease Control and Prevention, has made recommendations for identifying weakness and motor delay in children three months to five years of age.23 These recommendations are available at http://www.cdc.gov/ncbddd/musculardystrophy/diagnostic-tool.html.
Identification
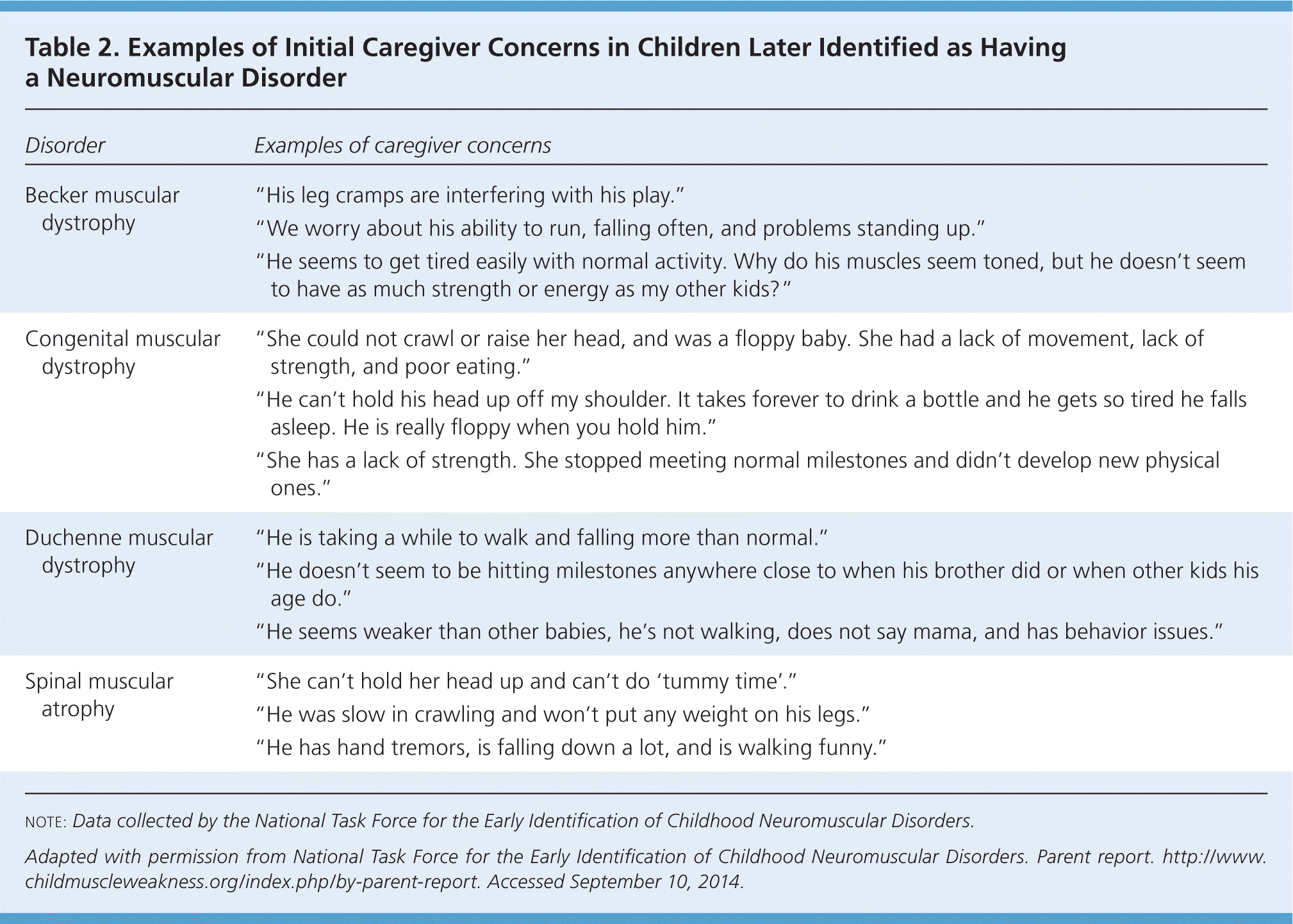
Children with neuromuscular disorders usually present with gross motor delays, although fine motor and cognitive delays may also be present. Identification starts with attention to caregiver concerns about the child's development (Table 2).24 Caregivers who express concern about developmental problems are correct more than 80% of the time.25–28 Caregivers may report concerns about a child's muscle tone, coordination, strength, and ambulation,24 as well as poor or slow feeding. During all well-child visits, clinicians should review developmental milestones, incorporating checklists or other tools (available at http://www.cdc.gov/ncbddd/actearly/hcp/index.html) and considering risk factors for neurologic dysfunction, such as prematurity.29,30 The use of screening tools completed by a caregiver, such as the Ages and Stages questionnaire31 or Parents' Evaluation of Developmental Status,32 before well-child visits adds little time to the length of the visit and improves sensitivity for identifying developmental delay (70% to 80%) with reasonable specificity (70% to 80%),33 comparing favorably to the estimated 30% sensitivity of clinical impression alone.20
| Disorder | Examples of caregiver concerns |
|---|---|
| Becker muscular dystrophy | “His leg cramps are interfering with his play.” |
| “We worry about his ability to run, falling often, and problems standing up.” | |
| “He seems to get tired easily with normal activity. Why do his muscles seem toned, but he doesn't seem to have as much strength or energy as my other kids?” | |
| Congenital muscular dystrophy | “She could not crawl or raise her head, and was a floppy baby. She had a lack of movement, lack of strength, and poor eating.” |
| “He can't hold his head up off my shoulder. It takes forever to drink a bottle and he gets so tired he falls asleep. He is really floppy when you hold him.” | |
| “She has a lack of strength. She stopped meeting normal milestones and didn't develop new physical ones.” | |
| Duchenne muscular dystrophy | “He is taking a while to walk and falling more than normal.” |
| “He doesn't seem to be hitting milestones anywhere close to when his brother did or when other kids his age do.” | |
| “He seems weaker than other babies, he's not walking, does not say mama, and has behavior issues.” | |
| Spinal muscular atrophy | “She can't hold her head up and can't do ‘tummy time’.” |
| “He was slow in crawling and won't put any weight on his legs.” | |
| “He has hand tremors, is falling down a lot, and is walking funny.” |
Evaluation
If developmental concerns are identified, more formal assessment is indicated. Because of the importance of early intervention, clinicians should not take a “wait and see” approach without further discussion or evaluation.34 It is imperative to consider other medical problems that could cause weakness and mimic neuromuscular disorders, particularly those that are potentially treatable, such as hypothyroidism, congenital heart disease, and nutritional deficiency.
During the focused evaluation of a child with weakness, the first objective is to establish whether the motor problem is primarily central (acquired brain injury, such as cerebral palsy) or peripheral (neuromuscular; involving the anterior horn cell, nerve, neuromuscular junction, or muscle). Central motor problems emerge without progressive weakness, whereas peripheral motor problems are degenerative conditions in which expected development lags or regresses. History might clarify the reasons for delay in cases of pre- and perinatal trauma or neonatal disease. Family history should be reviewed for weakness or developmental delay.
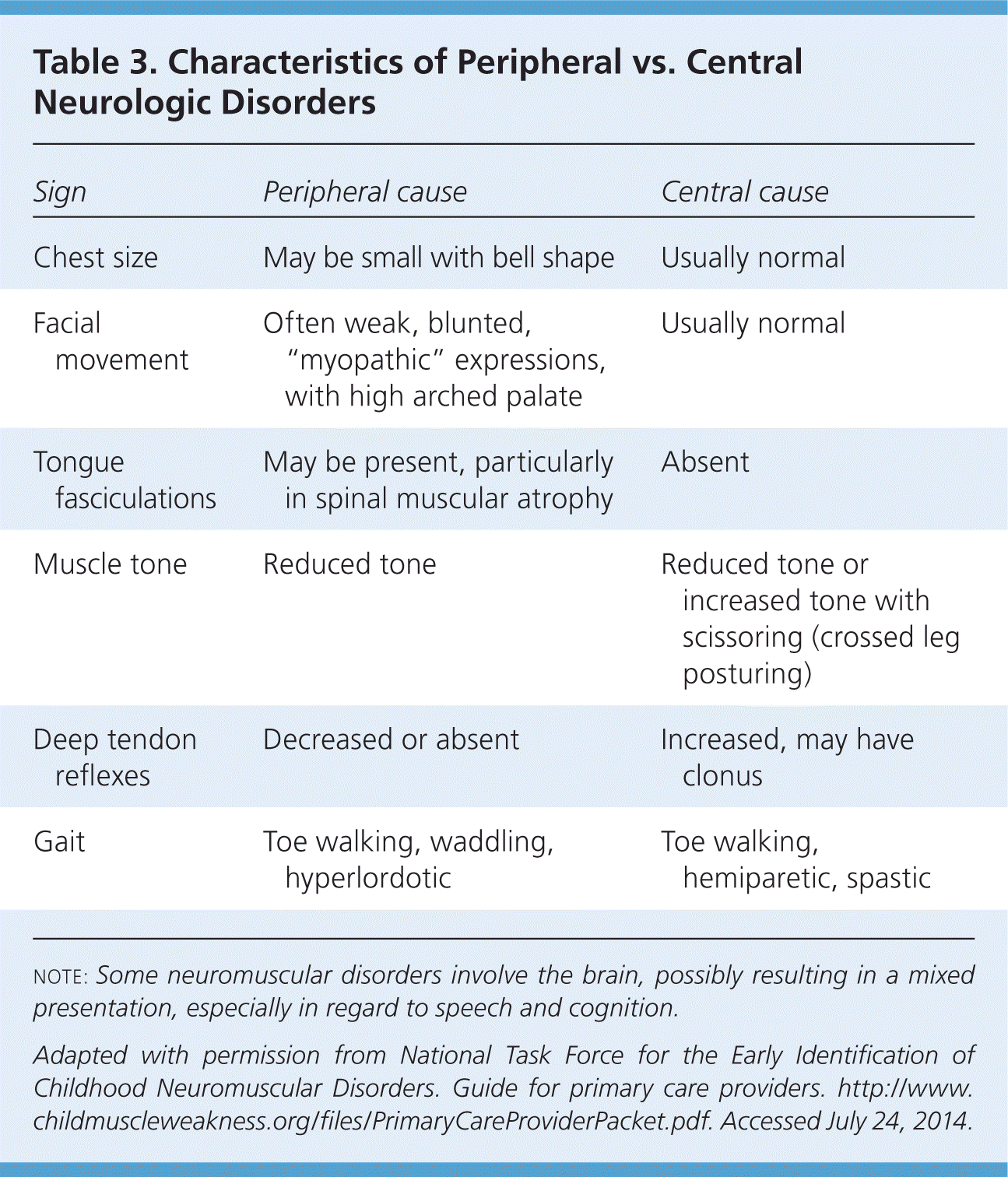
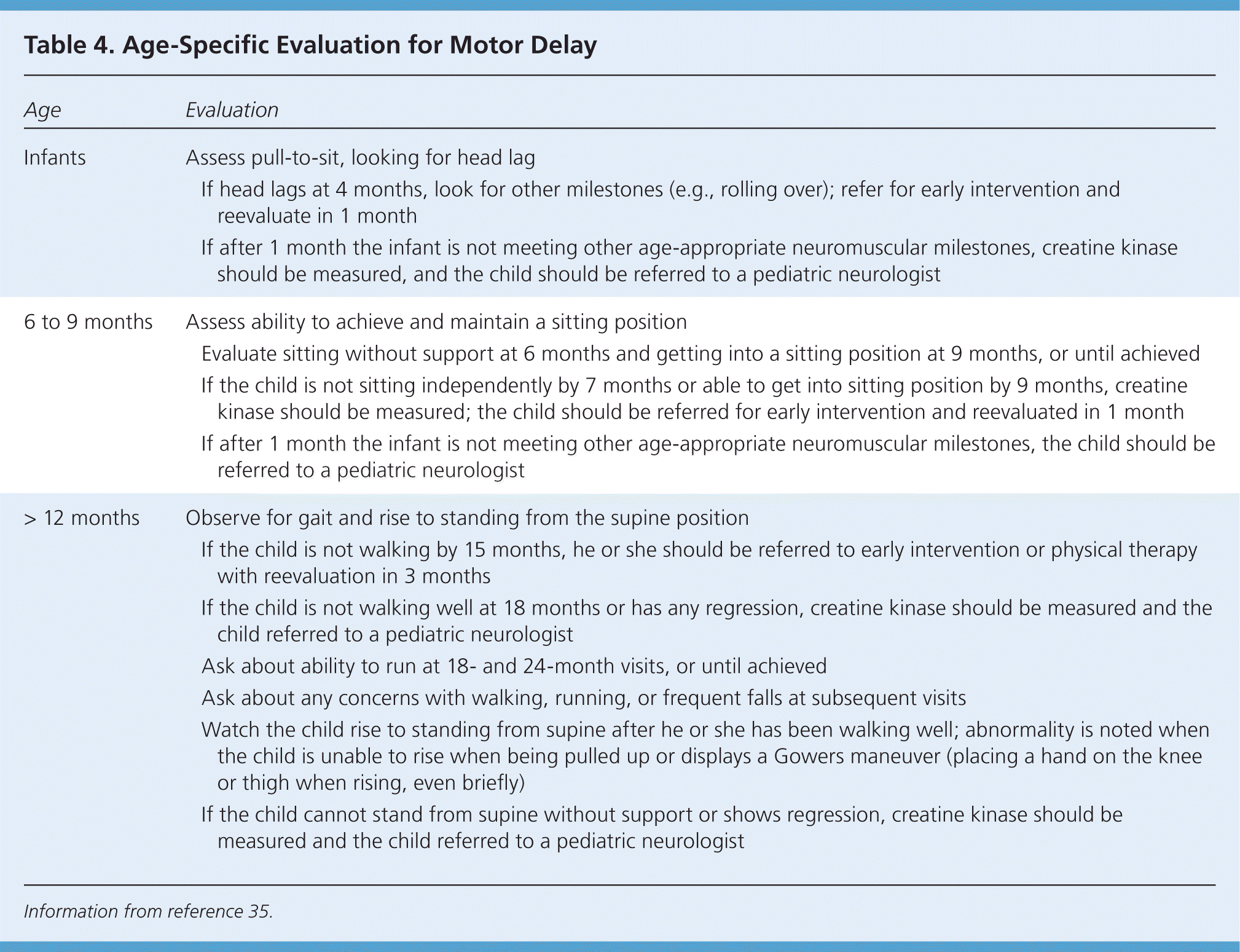
Physical examination confirms the presence and severity of weakness and can also help distinguish central from peripheral causes (Table 3).35 Special attention should be given to an age-appropriate motor evaluation, such as observing for head lag on pull-to-sit, independent sitting, and getting into the sitting position; standing from the floor; walking; and running. Placing the child on a floor mat helps in the evaluation of gross motor function. Recommendations for the age-specific examination are included in Table 4.35 Video clips of children with neuromuscular disorders demonstrating abnormal tone and strength are available at http://www.childmuscleweakness.org/index.php/videos.
| Sign | Peripheral cause | Central cause |
|---|---|---|
| Chest size | May be small with bell shape | Usually normal |
| Facial movement | Often weak, blunted, “myopathic” expressions, with high arched palate | Usually normal |
| Tongue fasciculations | May be present, particularly in spinal muscular atrophy | Absent |
| Muscle tone | Reduced tone | Reduced tone or increased tone with scissoring (crossed leg posturing) |
| Deep tendon reflexes | Decreased or absent | Increased, may have clonus |
| Gait | Toe walking, waddling, hyperlordotic | Toe walking, hemiparetic, spastic |
| Age | Evaluation | |
|---|---|---|
| Infants | Assess pull-to-sit, looking for head lag | |
| If head lags at 4 months, look for other milestones (e.g., rolling over); refer for early intervention and reevaluate in 1 month | ||
| If after 1 month the infant is not meeting other age-appropriate neuromuscular milestones, creatine kinase should be measured, and the child should be referred to a pediatric neurologist | ||
| 6 to 9 months | Assess ability to achieve and maintain a sitting position | |
| Evaluate sitting without support at 6 months and getting into a sitting position at 9 months, or until achieved | ||
| If the child is not sitting independently by 7 months or able to get into sitting position by 9 months, creatine kinase should be measured; the child should be referred for early intervention and reevaluated in 1 month | ||
| If after 1 month the infant is not meeting other age-appropriate neuromuscular milestones, the child should be referred to a pediatric neurologist | ||
| > 12 months | Observe for gait and rise to standing from the supine position | |
| If the child is not walking by 15 months, he or she should be referred to early intervention or physical therapy with reevaluation in 3 months | ||
| If the child is not walking well at 18 months or has any regression, creatine kinase should be measured and the child referred to a pediatric neurologist | ||
| Ask about ability to run at 18- and 24-month visits, or until achieved | ||
| Ask about any concerns with walking, running, or frequent falls at subsequent visits | ||
| Watch the child rise to standing from supine after he or she has been walking well; abnormality is noted when the child is unable to rise when being pulled up or displays a Gowers maneuver (placing a hand on the knee or thigh when rising, even briefly) | ||
| If the child cannot stand from supine without support or shows regression, creatine kinase should be measured and the child referred to a pediatric neurologist | ||
Testing
LABORATORY
When the evaluation suggests a peripheral neuromuscular problem, serum creatine kinase (CK) levels should be measured.36 The measurement is rarely elevated with primary central nervous system disease, whereas it is markedly elevated in many muscular dystrophies (e.g., Duchenne muscular dystrophy) and mildly elevated or normal in spinal muscular atrophy, neuropathies, congenital myopathies, and some other muscular dystrophies. eTable A summarizes general guidelines for interpretation of serum CK measurements. If the CK level is mildly elevated, testing should be repeated in two to three weeks. Levels that are grossly (more than three times the upper limit of normal) or persistently elevated warrant referral to a pediatric neurologist.35
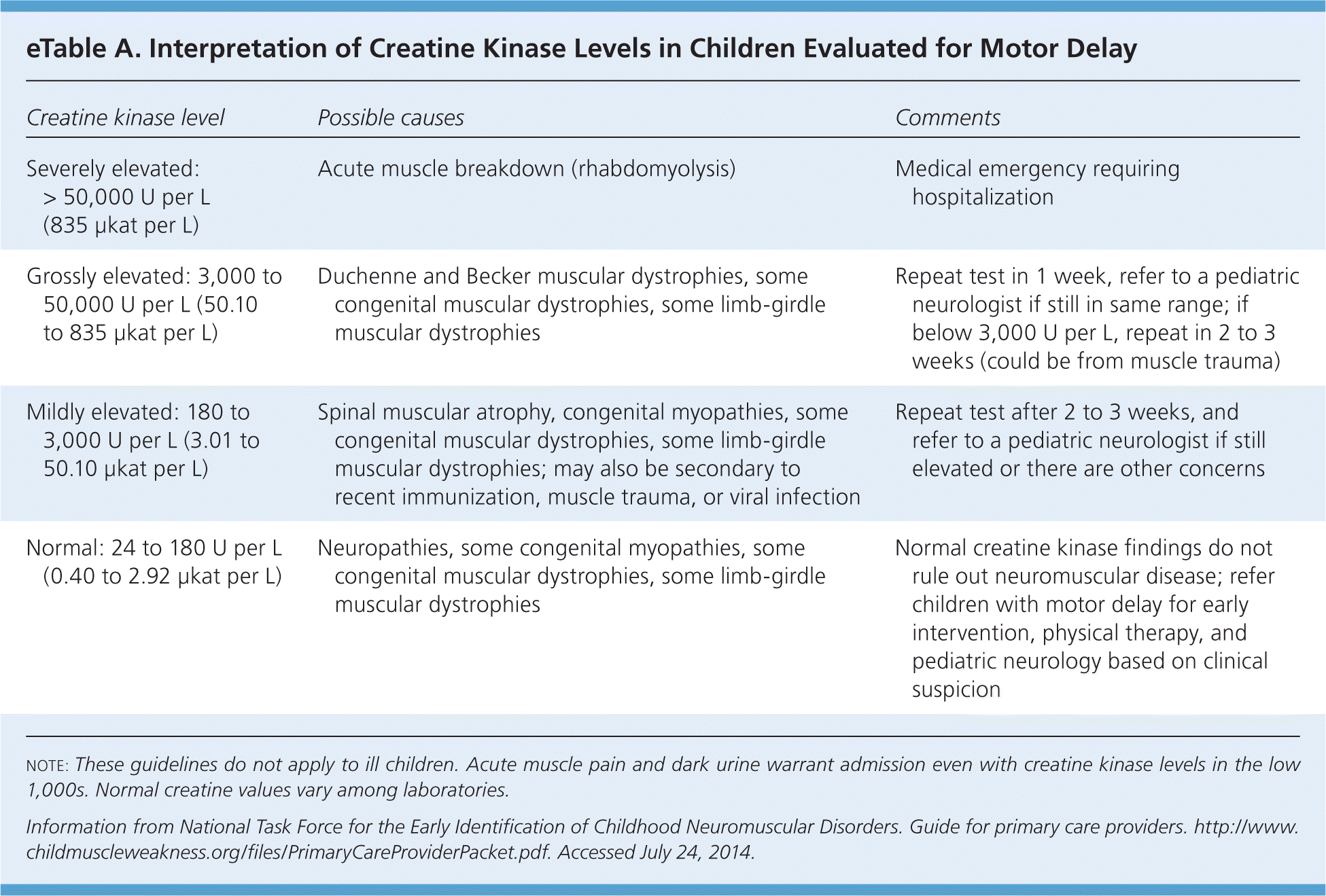
| Creatine kinase level | Possible causes | Comments |
|---|---|---|
| Severely elevated: > 50,000 U per L (835 μkat per L) | Acute muscle breakdown (rhabdomyolysis) | Medical emergency requiring hospitalization |
| Grossly elevated: 3,000 to 50,000 U per L (50.10 to 835 μkat per L) | Duchenne and Becker muscular dystrophies, some congenital muscular dystrophies, some limb-girdle muscular dystrophies | Repeat test in 1 week, refer to a pediatric neurologist if still in same range; if below 3,000 U per L, repeat in 2 to 3 weeks (could be from muscle trauma) |
| Mildly elevated: 180 to 3,000 U per L (3.01 to 50.10 μkat per L) | Spinal muscular atrophy, congenital myopathies, some congenital muscular dystrophies, some limb-girdle muscular dystrophies; may also be secondary to recent immunization, muscle trauma, or viral infection | Repeat test after 2 to 3 weeks, and refer to a pediatric neurologist if still elevated or there are other concerns |
| Normal: 24 to 180 U per L (0.40 to 2.92 μkat per L) | Neuropathies, some congenital myopathies, some congenital muscular dystrophies, some limb-girdle muscular dystrophies | Normal creatine kinase findings do not rule out neuromuscular disease; refer children with motor delay for early intervention, physical therapy, and pediatric neurology based on clinical suspicion |
Elevated serum transaminase levels in the context of motor delay should also trigger CK testing. Because aspartate transaminase and alanine transaminase can come from muscle or the liver and CK comes only from muscle, CK testing can prevent unnecessary liver evaluation.
Identifying elevated CK levels may be lifesaving. Some neuromuscular disorders increase the risk of malignant hyperthermia with the use of inhalation anesthetics. Because weak children are at risk of fracture37 and may have an increased need for surgery, knowledge of this risk is particularly pertinent.
It is reasonable to measure thyroxine and thyroid-stimulating hormone levels, especially if routine newborn test results are unavailable, because congenital hypothyroidism can cause muscle weakness.38
IMAGING
Radiography is not indicated in the evaluation of neuromuscular delay, although a neuromuscular disorder may be initially suspected because of the bell shape on chest radiography or a scoliosis curve in a C shape as opposed to an S shape.39 Although brain or spine magnetic resonance imaging is indicated for children with increased tone and suspected cerebral palsy, micro- or macrocephaly, or neurocognitive delay or regression,40 it is not a routine component of the evaluation of a child with isolated weakness.
Referral and Follow-Up
Figure 1 is an algorithm for the appropriate referral of children with suspected neuromuscular disorders.35 In most cases, referral to an early intervention program is recommended whenever developmental delay is identified.41 These programs provide comprehensive evaluation and, when appropriate, services directed at improving motor, cognitive, speech, or social problems. Involvement in an early intervention program can also provide caregivers with emotional support and respite time. Children who have problems with motor function may benefit from physical and/or occupational therapy.
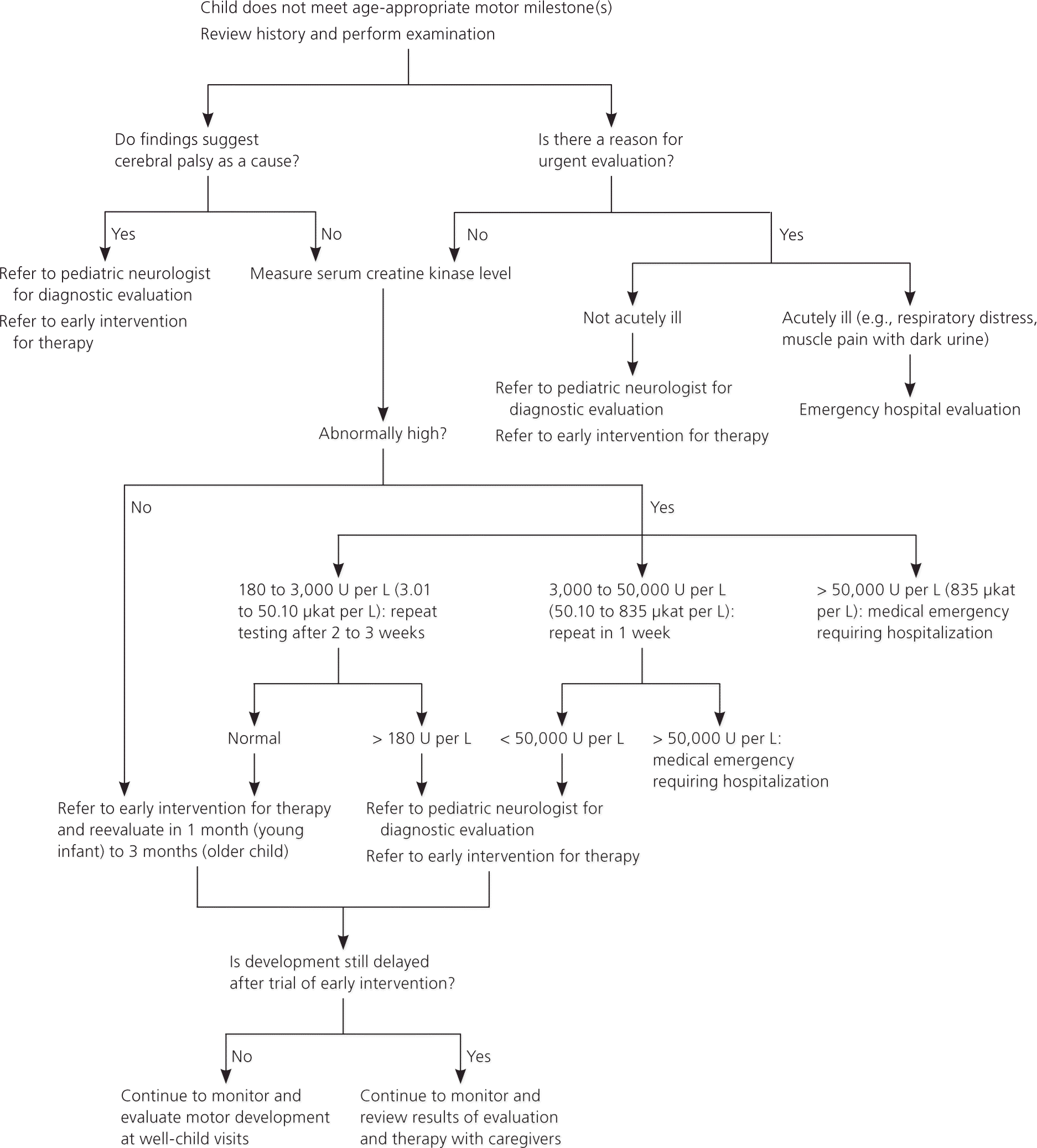
If a child is referred to an early intervention program, primary care follow-up appointments should be scheduled in one month for infants and in two to three months for younger children. If progress toward developmental milestones remains poor, the child should be referred to a pediatric neurologist. While the neurology evaluation is in progress, therapists should avoid using strengthening exercises that might be counterproductive in patients who have some neuromuscular disorders with high CK levels.
Although neuromuscular delay is rarely an emergency, red flag indications for immediate neurology referral include presence of tongue fasciculations (suggesting spinal muscular atrophy), loss of motor milestones, or a CK level greater than three times the upper limit of normal. Children with neuromuscular weakness are at increased risk of respiratory failure with infection or from fatigue. Clinicians should have a low threshold for hospitalization and monitoring of blood gases for carbon dioxide retention if a weak child has signs or symptoms of respiratory distress.
Role of the Family Physician
The family physician can help the family navigate subspecialty visits and consultations, advocate for services in the school and home, and help them cope with the emotional stresses of caring for a child with special needs. More frequent primary care visits (in addition to routine well-child visits) after subspecialty referrals can be used to monitor progress and test results, and to support and assist families. It is important to ask caregivers how they feel when the problem is identified and again during follow-up. Caregivers often have conflicting emotions: relief that their concerns were heard, but worry that the child has a medical condition. Sometimes anger will be directed at the clinician for bringing unwelcome news. It is helpful to use active listening techniques, such as acknowledging the caregiver's feeling by paraphrasing what he or she has said and using the BATHE (background, affect, troubles, handling, empathy) protocol.42,43
Caregivers should be reassured that even the most serious neuromuscular disorders are unlikely to suddenly and dramatically worsen. A clear plan for evaluation and follow-up and description of which health care professionals will be involved can help minimize worry. Caregivers should be given the opportunity to respond to this plan, including whether they understand the plan and are able to follow it.
The whole family should be considered. Not only can siblings feel neglected by parents who devote a disproportionate amount of time to the affected child, but the parents can also find themselves isolated from peers whose children are developing normally. Separation or divorce of parents who have a child with special needs is not uncommon.44,45 Family physicians should be aware of the stress and burden neuromuscular disorders place on caregivers and other family members, and should be prepared to provide or facilitate mental health services. Table 5 provides resources that may be helpful to families.

| Cure CMD (congenital muscular dystrophy) | |
| http://curecmd.org | |
| Cure SMA (spinal muscular atrophy) | |
| http://curesma.org | |
| Muscular Dystrophy Association | |
| http://mdausa.org | |
| Parent Project Muscular Dystrophy: for Duchenne and Becker muscular dystrophies | |
| http://www.parentprojectmd.org | |
| Spinal Muscular Atrophy Foundation | |
| http://www.smafoundation.org | |
Data Sources: We searched Medline using the key words weakness, neuromuscular, muscular dystrophy, and spinal muscular atrophy. Additional searches were performed using Essential Evidence Plus, the Agency for Healthcare Research and Quality clinical guidelines and evidence reports, Cochrane Database of Systematic Reviews, Google Scholar, DynaMed, and UpToDate. Search dates: June and October 2013. We repeated our search using DynaMed, Academic Search Complete, EBSCOhost, and Cochrane Database of Systematic Reviews. Search date: September 1, 2014.
The authors are members of the National Task Force for the Early Identification of Childhood Neuromuscular Disorders, which is partially supported by the National Center on Birth Defects and Developmental Disabilities at the Centers for Disease Control and Prevention. This paper, and its conclusions and recommendations, are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
