
Am Fam Physician. 2015;91(10):692-697
Patient information: See related handout on severe heartburn (GERD), written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Common questions that arise regarding treatment of gastroesophageal reflux disease (GERD) include which medications are most effective, when surgery may be indicated, which patients should be screened for Barrett esophagus and Helicobacter pylori infection, and which adverse effects occur with these medications. Proton pump inhibitors (PPIs) are the most effective medical therapy, and all PPIs provide similar relief of GERD symptoms. There is insufficient evidence to recommend testing for H. pylori in patients with GERD. In the absence of alarm symptoms, endoscopy is not necessary to make an initial diagnosis of GERD. Patients with alarm symptoms require endoscopy. Screening for Barrett esophagus is not routinely recommended, but may be considered in white men 50 years or older who have had GERD symptoms for at least five years. Symptom remission rates in patients with chronic GERD are similar in those who undergo surgery vs. medical management. PPI therapy has been associated with an increased risk of hip fracture, hypomagnesemia, community-acquired pneumonia, vitamin B12 deficiency, and Clostridium difficile infection.
More than 60 million persons in the United States report symptoms of gastroesophageal reflux disease (GERD) at least weekly, and a typical full-time family physician can expect to diagnose and treat 40 to 60 patients with this condition each month.1 Three medication classes are available for prescription and over-the-counter treatment: proton pump inhibitors (PPIs), histamine H2 receptor antagonists, and antacids.2 Although endoscopy is not necessary to diagnose GERD in most patients, endoscopic screening for complications of GERD is warranted when alarm symptoms are present (e.g., involuntary weight loss, anemia, evidence of bleeding or obstruction, dysphagia, persistent symptoms despite adequate medical therapy) or in patients 50 years and older.3,4 This article reviews common questions that arise in the management of GERD.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| There are no significant differences among equivalent doses of PPIs for the treatment of nonerosive GERD. | A | 2 |
| Anti-reflux surgery should generally be reserved for patients with contraindications to PPI therapy or when PPI therapy alone is insufficient to control symptoms. | C | 15 |
| Screening for Barrett esophagus is not routinely recommended in patients with GERD, but it may be considered in white men 50 years or older who have had GERD symptoms for at least five years. | C | 4, 16 |
| Endoscopy should be limited to patients who have alarm symptoms or persistent GERD symptoms after an adequate trial of PPI therapy. | C | 31 |
| Testing for Helicobacter pylori in patients with GERD is not recommended. | C | 40, 41 |

| Recommendation | Sponsoring organization |
|---|---|
| Long-term acid suppression therapy for gastroesophageal reflux disease should be titrated to the lowest effective dose. | American Gastroenterological Association |
Are All PPIs Equally Effective in Relieving GERD Symptoms? What Is Their Effectiveness Compared with, or Combined with, Other Medications?
All over-the-counter and prescription PPIs offer similar relief of GERD symptoms; therefore, physicians should choose the appropriate PPI based on cost, formulary availability, and patient response.2,5 PPIs are more effective for relieving GERD symptoms than H2antagonists, and may be more cost-effective than step therapy. In one randomized controlled trial (RCT), domperidone (not available in the United States) plus omeprazole (Prilosec) was superior to omeprazole alone6 ; however, the overall evidence for adding prokinetics to PPI therapy is inconclusive.
EVIDENCE SUMMARY
A recent Cochrane review identified three trials of treatments for nonerosive GERD.7 These trials compared equivalent doses of four PPIs: esomeprazole (Nexium) 20 mg, omeprazole 20 mg, pantoprazole (Protonix) 20 mg, and rabeprazole (Aciphex) 10 mg. All had similar times to initial relief of symptoms and complete relief of symptoms at four weeks. Although a meta-analysis concluded that high doses of esomeprazole were slightly superior to other PPIs in healing erosive GERD at eight weeks (absolute risk reduction = 4%; number needed to treat [NNT] = 25),8 erosive esophagitis is present in a minority of patients who undergo endoscopy for GERD (23% of participants in one study).1
One RCT supported switching PPIs if the initial choice was ineffective.9 A Cochrane review found that PPIs are more effective at relieving GERD symptoms than H2 antagonists,7 and an RCT supported the cost- and clinical effectiveness of starting with a PPI rather than step therapy with an H2 antagonist when treating reflux esophagitis.10
Adding prokinetic medications to PPI therapy may be an option in patients with chronic GERD. One RCT comparing omeprazole 20 mg twice daily plus domperidone 10 mg three times daily vs. omeprazole 20 mg twice daily found decreased symptoms with a mean improvement using a validated symptom score (Frequency Scale for Symptoms of GERD; P = .02).6 However, a meta-analysis of 12 RCTs found that the combination of a prokinetic and PPI did not improve symptoms and was associated with more adverse effects than a PPI alone.11
When Should a Patient with GERD Be Referred for Surgery?
Surgery should be reserved for patients with contraindications to PPI therapy or when symptoms remain poorly controlled despite lifestyle changes and maximal PPI doses. Medical treatment should be optimized, medication adherence should be addressed, and risks associated with surgery should be carefully considered when weighing treatment options.
EVIDENCE SUMMARY
Surgical options for GERD include laparoscopic or open Nissen fundoplication. A five-year, randomized, open parallel group trial compared long-term esomeprazole use with laparoscopic anti-reflux surgery. The authors found a clinically significant difference in symptom remission rates (92% [90% confidence interval (CI), 89% to 96%] in the esomeprazole group vs. 85% [95% CI, 81% to 90%] in the surgery group; NNT = 14; P = .048).12,13 Surgery is usually reserved for patients who are unable or unwilling to take PPIs and for those with inadequate symptom control despite maximal dosing and compliance with PPI treatment.10 In this population, surgery is cost-effective and is associated with a high quality of life with fewer heartburn days three years later.14
Patients who do not respond to PPIs generally have poor surgical outcomes.3 Postoperative dysphagia, bloating, and a short-term increase in mortality are common complications of anti-reflux surgery.3 There is insufficient evidence that anti-reflux surgery improves outcomes for patients with Barrett esophagus.15 Several endoscopic and laparoscopic alternatives to fundoplication have been tested but have limited effectiveness.
Which Patients with GERD Should Be Screened for Barrett Esophagus?
Current guidelines suggest individualized screening in certain populations at greater risk of Barrett esophagus based on observational studies of varying quality. Screening for Barrett esophagus appears to be cost-effective in white men 50 years or older who have had GERD symptoms for at least five years.4,16
EVIDENCE SUMMARY
Although up to 10% of patients with chronic reflux symptoms will have Barrett esophagus,17 the annual risk of progression to esophageal adenocarcinoma is low (approximately 0.12% to 0.33% per year).18 Screening for Barrett esophagus based on GERD symptoms alone is likely to miss up to 50% of patients with this premalignant condition.17 Two large prospective studies examining surveillance programs for patients with Barrett esophagus did not demonstrate survival benefit.19,20 Persons at highest risk of Barrett esophagus include smokers with self-reported weekly GERD symptoms (odds ratio [OR] = 51.4), and those with body mass index greater than 30 kg per m2 with self-reported weekly GERD symptoms (OR = 34.4). In addition, a family history of Barrett esophagus21,22 and a history of severe, erosive esophagitis23–25 are important risk factors that should prompt endoscopic screening for Barrett esophagus (Table 119,26–29 ).
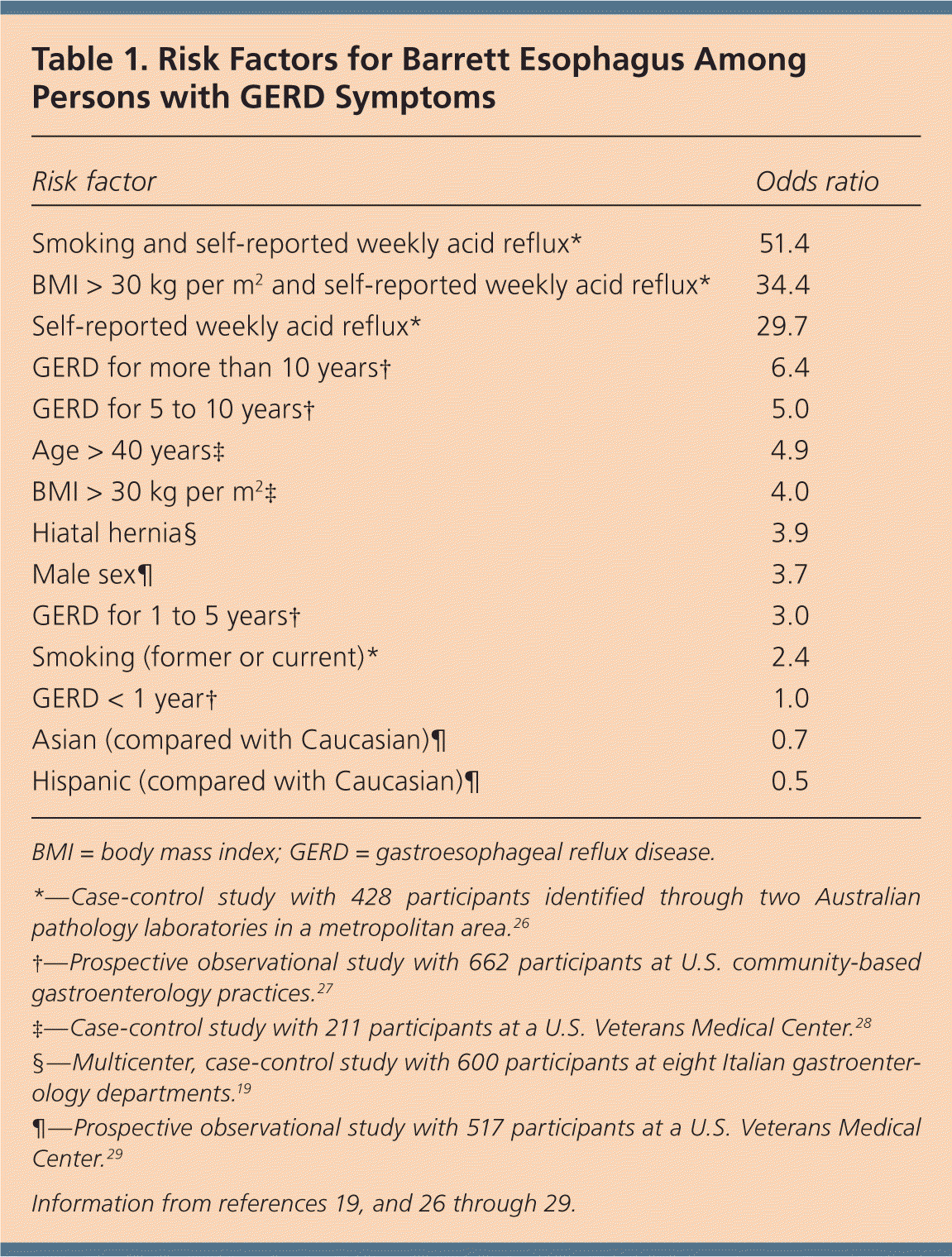
| Risk factor | Odds ratio |
|---|---|
| Smoking and self-reported weekly acid reflux* | 51.4 |
| BMI > 30 kg per m2 and self-reported weekly acid reflux* | 34.4 |
| Self-reported weekly acid reflux* | 29.7 |
| GERD for more than 10 years† | 6.4 |
| GERD for 5 to 10 years† | 5.0 |
| Age > 40 years‡ | 4.9 |
| BMI > 30 kg per m2 ‡ | 4.0 |
| Hiatal hernia§ | 3.9 |
| Male sex¶ | 3.7 |
| GERD for 1 to 5 years† | 3.0 |
| Smoking (former or current)* | 2.4 |
| GERD < 1 year† | 1.0 |
| Asian (compared with Caucasian)¶ | 0.7 |
| Hispanic (compared with Caucasian)¶ | 0.5 |
How Should GERD Be Diagnosed?
Initial diagnosis of GERD is based on typical symptoms such as heartburn or regurgitation. Endoscopy is not indicated in the absence of alarm symptoms. The diagnosis of GERD should be considered in nonsmoking patients with chronic cough of more than three weeks' duration. A four- to eight-week trial of a PPI is recommended before endoscopy is considered. Diagnosis of GERD can be improved using validated diagnostic tools such as the GerdQ questionnaire and a Danish prediction score for response to PPIs. All patients with alarm symptoms require endoscopic evaluation.
EVIDENCE SUMMARY
Typical symptoms of heartburn and regurgitation will correctly identify 70% of patients with GERD,30 and patients with these symptoms should receive an empiric trial of PPI therapy for four to eight weeks. This recommendation is based on the low risk associated with uncomplicated GERD and the ineffectiveness of screening and prevention of esophageal adenocarcinoma, which is the major source of morbidity and mortality in patients with GERD. In addition, overall mortality from other complications of GERD is low (0.46 per 100,000 cases in 2000).31 GERD should be considered in nonsmoking patients with chronic cough of at least three weeks' duration; prospective cohort studies show that the condition is present in up to 40% of these patients.32,33
All patients with alarm symptoms require endoscopic evaluation. Individual symptoms of involuntary weight loss, dysphagia, and anemia are specific (84%, 85%, and 95%, respectively) for complications such as esophageal or stomach cancer, bleeding foregut lesions, or esophageal or pyloric stenosis.34
Patients who do not respond to once-daily dosing should be switched to twice-daily dosing or a trial of a different PPI, and physicians should stress compliance with the regimen and the importance of taking the medication 30 to 60 minutes before meals.3,4 If medication timing is problematic, dexlansoprazole (Dexilant) can be considered because it has similar effectiveness regardless of meal timing. Also, patients with significant nighttime reflux symptoms may benefit from omeprazole/sodium bicarbonate (Zegerid) because of its effectiveness in controlling nighttime pH. Endoscopy should be limited to patients who do not respond to appropriate PPI therapy.
Although there are several symptom scores for diagnosing GERD, the six-question GerdQ diagnostic score was developed and validated in primary care patients with upper gastrointestinal symptoms.35 An online version is available at https://www.aafp.org/afp/2010/0515/p1278.html#afp20100515p1278-t1. This tool increases diagnostic accuracy by ruling out patients with scores of 0 to 2 and correctly identifies GERD in 80% of patients with scores of 8 or above.35 A second tool was designed and validated in 471 patients in a Danish multicenter trial.36 Nighttime pain, absence of nausea, and use of antacids or H2 antagonists in the previous month by average-weight to overweight patients significantly increased response to omeprazole. The use of these tools may help enhance diagnostic accuracy and decrease inappropriate endoscopy.
Should Patients with GERD Be Tested for H. pylori?
There is insufficient evidence to routinely test for Helicobacter pylori in patients with GERD. Although H. pylori is sometimes present, and eradicating it may improve symptoms, a subset of patients with peptic ulcer disease may have worsening of GERD symptoms or the development of new GERD symptoms after treatment for H. pylori infection.37
EVIDENCE SUMMARY
Because the symptoms of GERD, dyspepsia, and peptic ulcer disease often overlap, it is important to differentiate between these entities for effective treatment. Dyspepsia presents with pain centered in the upper abdomen or discomfort characterized by fullness, bloating, distension, or nausea, and can be associated with GERD. This is an important distinction because a “test and treat” strategy for H. pylori infection is recommended for patients with dyspepsia,38,39 but should not be used in all patients with GERD. One meta-analysis supports treating H. pylori infection in patients with GERD.40 This study found no increases in GERD symptoms or endoscopic evidence of reflux esophagitis after treatment for H. pylori infection, and a subgroup analysis of five trials found that eradication of H. pylori was associated with significant improvement in GERD symptoms (OR = 0.55; 95% CI, 0.35 to 0.87).40 However, another meta-analysis found a twofold higher risk of developing erosive GERD after H. pylori eradication in patients with peptic ulcer disease (OR = 2.04; 95% CI, 1.08 to 3.85), highlighting potential concerns about routine H. pylori testing and treatment in patients with GERD symptoms.41
What Are the Complications Associated with Long-Term PPI Use?
PPIs may increase the risk of hypomagnesemia, hip fracture, Clostridium difficile infection, vitamin B12deficiency, and community-acquired pneumonia. Therefore, PPIs should be used only when there is an appropriate diagnosis, at the lowest effective dose and shortest duration of therapy.
EVIDENCE SUMMARY
A previous article in American Family Physician reviewed adverse effects of long-term PPI use for various indications.42
Hypomagnesemia. A large retrospective, cross-sectional analysis in an ambulatory population found an increased incidence of hypomagnesemia and identified cases of severe hypomagnesemia in patients who had been treated with a PPI in the four months before testing (OR = 3.79; 95% CI, 2.99 to 4.82).43 The clinical significance of this finding is uncertain.
Hip Fracture. Several studies found an association between long-term PPI use and increased risk of hip fractures.44 However, a recent large case-control study found that those at risk of hip fracture were receiving higher doses of PPIs, and that the increased risk was confined to those with at least one additional risk factor (OR = 1.41; 95% CI, 1.21 to 1.64).44
C. difficile Infection. PPI use may increase susceptibility to C. difficile. In one systematic review, 17 of 27 studies showed an increased risk (risk ratio = 1.2 to 5.0).45 However, data are conflicting on the increased risk of recurrent C. difficile infection when PPIs are used during treatment. In one retrospective cohort study using Veterans Administration data, the risk of recurrent infection after initial treatment was increased by 42% in patients who received PPIs during the course of treatment (OR = 1.42; 95% CI, 1.11 to 1.82).46 However, in another RCT reviewing inpatient treatment of C. difficile infection, there was no increased risk of recurrence (hazard ratio = 0.82; 95% CI, 0.58 to 1.16).47
Vitamin B12Deficiency. A large case-control study indicated an increased risk of vitamin B12 deficiency in patients treated with PPIs (OR = 1.65; 95% CI, 1.58 to 1.73).48 Thus, patients with suggestive symptoms should be tested for vitamin B12 deficiency.
Data Sources: Essential Evidence Plus, PubMed, and the Cochrane Database of Systematic Reviews were searched using the following keywords: PPI and GERD treatment, Barrett's esophagus treatment, Barrett's esophagus management, surgical options for GERD, surgery versus PPI for GERD, pneumonia and PPI use, hip fractures and PPI use, hypomagnesaemia and PPI use, C. difficile and PPI use, and H. pylori and GERD. Search dates: May 2014 through July 2014.
