Am Fam Physician. 2015;91(12):828-833
Author disclosure: No relevant financial affiliations.
Dapagliflozin (Farxiga) is the first of a new class of medication used to treat type 2 diabetes mellitus by increasing urinary glucose excretion. It inhibits the sodium-glucose cotransporter 2 enzyme, working on the early proximal convoluted renal tubule to reduce the reuptake of glucose by 30% to 50%.1
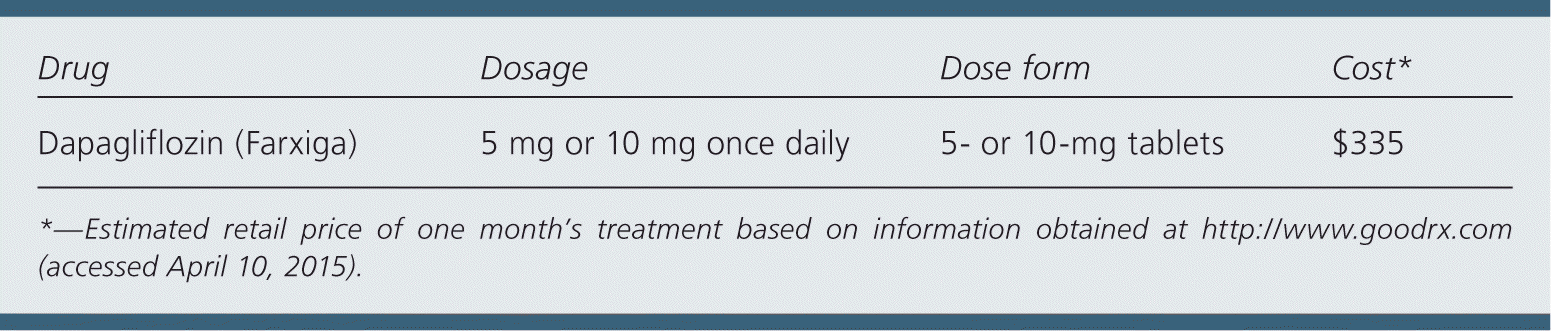
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Dapagliflozin (Farxiga) | 5 mg or 10 mg once daily | 5- or 10-mg tablets | $335 |
SAFETY
Because dapagliflozin has been shown to induce glycosuria, patients should be warned about the most common clinical sequelae of osmotic diuresis, hypotension, and hypoglycemia. Systolic blood pressure typically decreases by 2.3 to 5.6 mm Hg. However, hypotension is relatively uncommon (number needed to harm [NNH] = 337) and mainly affects older adults, patients on loop diuretics, and those with renal impairment. Hypoglycemia occurs infrequently overall (NNH = 133). Among patients also taking insulin, the risk of mild or moderate hypoglycemia increases (NNH = 20), but not the rate of major hypoglycemic episodes, defined as those requiring external assistance.2 This class of medication has also been linked to ketosis and diabetic ketoacidosis without the associated usual high levels of blood glucose.3
During phase 2 and 3 clinical trials,4,5 an imbalance was noted in the number of cases of bladder cancer (207 vs. 53 cases per 100,000 person-years in the intervention and control groups, respectively). However, detection bias may be a contributor, because three patients had microscopic hematuria at baseline, and those in the intervention group may have had more frequent urinalyses because of glycosuria.4 Dapagliflozin should not be used in patients with known bladder cancer, and patients should be told to notify their physician if they experience any hematuria.
No human studies have included pregnant or breastfeeding mothers.6 This medication is U.S. Food and Drug Administration pregnancy class C and should be avoided in the late second and third trimesters during fetal renal development.
TOLERABILITY
Dapagliflozin is generally well tolerated, with only 0.2% of patients in premarketing trials discontinuing treatment because of adverse effects. Women taking dapagliflozin are more likely to develop vulvovaginal candidiasis (6.9% to 8.5%; NNH = 15 to 19) and men are more likely to develop balanitis (2.7% to 2.8%; NNH = 40 to 42) compared with patients taking placebo.7 There is a higher risk of urinary tract infections as well (NNH = 50).8 Use of dapagliflozin will increase total cholesterol by an average of 2.5% and low-density lipoprotein cholesterol by 2.9%, which may affect the need for statin therapy.
EFFECTIVENESS
There are no data on the morbidity or mortality benefits of long-term use. In patients who take dapagliflozin (10 mg once daily) as monotherapy, A1C levels decrease an average of 0.7 percentage points more than placebo (95% confidence interval, 0.4% to 1.0% lower). The overall decrease in A1C level is less than that with metformin and insulin, but similar to that of other newer diabetic agents. When dapagliflozin is used in conjunction with metformin, A1C levels decrease by 0.5 percentage points more than with either dapagliflozin or metformin alone. When compared head-to-head against a sulfonylurea as a part of dual-agent therapy with metformin, dapagliflozin demonstrates an equal decrement in A1C measurements. Patients will lose an average of 2% body weight (about 2.4 lb [1.1 kg]), which may be caused by loss of excessive energy in the form of renal glucose excretion. It is unclear how long this weight loss persists, because it has not been studied for a period of more than 52 weeks.8 Dapagliflozin is less effective in patients with renal dysfunction because less glucose is cleared renally in this group. Urine glucose levels should not be used to monitor its effectiveness.
PRICE
A one-month supply of dapagliflozin costs approximately $335. This is significantly more expensive than metformin or glipizide (Glucotrol), which cost about $4 for a one-month supply.
SIMPLICITY
The recommended starting dosage is 5 or 10 mg once daily in the morning (to prevent nocturia), taken with or without food.
Bottom Line
Dapagliflozin will lower A1C levels by an average of 0.7 percentage points when used alone or by an average of 0.5 percentage points when added to metformin therapy. Its effect on diabetes-associated morbidity and mortality has not been studied, and although it is well tolerated, there is an association with bladder cancer and diabetic ketoacidosis. It is also considerably more expensive than other metformin add-on options.7,8 Consider dapagliflozin as a third-line oral agent, after initiating metformin therapy and a sulfonylurea, for patients who are at low risk of developing genitourinary infections and bladder cancer.