
A more recent article on routine immunizations for children and adolescents is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2015;92(6):460-468
Related editorials: HPV Vaccination: Overcoming Parental and Physician Impediments and Navigating the Changes in Pneumococcal Immunizations for Adults
Author disclosure: No relevant financial affiliations.
Recommendations for routine vaccinations in children and adolescents have changed multiple times in recent years, based on findings in clinical trials, licensure of new vaccines, and evidence of waning immunity. Despite the overwhelming success of vaccinations, vaccine delay and refusal are leading to pockets of vaccine-preventable diseases. Schedules for diphtheria and tetanus toxoids, and acellular pertussis (DTaP); hepatitis A and B; Haemophilus influenzae type b (Hib); inactivated poliovirus; varicella; and measles, mumps, and rubella are unchanged. However, since 2008, 13-valent pneumococcal conjugate vaccine has replaced the 7-valent vaccine; a new two-dose oral rotavirus vaccine has been approved; use of the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine has been expanded to children seven to 10 years of age who received fewer than five doses of DTaP, as well as during each pregnancy; a booster dose of meningococcal vaccine is recommended in adolescents 16 to 18 years of age (unless the first dose was given after 16 years of age); new meningococcal vaccines have been approved for use in infants at high risk of meningococcal disease; influenza vaccine has been expanded to routine use in all children six months and older; and the human papillomavirus vaccine has been approved for routine immunization of adolescent boys and girls. For the 2015–2016 influenza season, either live attenuated or inactivated vaccine can be administered to healthy children two to eight years of age.
High vaccine coverage is one of the major public health achievements in recent years, particularly with regard to nearly eliminating and dramatically decreasing the 13 vaccine-preventable diseases for which vaccinations were in place before 2005.1,2 For children born in the United States from 1994 to 2013, vaccination will prevent an estimated 322 million illnesses, 21 million hospitalizations, and 732,000 deaths during their lifetimes.3 Coverage for most vaccines for children 19 to 35 months of age has been stable since 2012, with the exception of a slight increase in rotavirus and birth-dose hepatitis B vaccinations, and a decline in the rates of Haemophilus influenzae type b (Hib) vaccination from 2005 to 2010, possibly related to vaccine shortages.4,5 According to the National Immunization Survey–Teen (2007 to 2013), vaccination coverage for adolescents is improving, although still behind the Healthy People 2020 goals for human papillomavirus (HPV) and meningococcal vaccinations.6,7 Evidence-based findings to improve vaccine access in communities, encourage community demand for vaccinations, and encourage physicians and health care systems to provide vaccines have contributed to many immunization rates reaching the Healthy People 2020 goals (Table 1).4,6 Recommendations from the Community Preventive Services Task Force include many interventions that improve vaccination rates (Table 2).6,8
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Physicians should explain to parents that vaccines—including the measles, mumps, and rubella vaccine—are beneficial, safe, and effective. | C | 28, 34 |
| Physicians should reassure parents that there is no evidence that vaccines cause autism or neurologic problems. | C | 28, 35 |
| Physicians should inform parents that the risk of intussusception with the rotavirus vaccine is minimal compared with the decrease in morbidity and mortality associated with rotavirus diarrheal disease. | C | 38–40 |
| Live attenuated influenza vaccine and inactivated influenza vaccine are both appropriate options in healthy children two to eight years of age who have no contraindications. Either vaccine is appropriate in older children and in adults up to 49 years of age. | C | 27, 48, 51 |
| The tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine should be administered to pregnant women at 27 to 36 weeks' gestation to provide passive immunity for their infants. | C | 57, 58 |
| Human papillomavirus vaccine should be administered to adolescent females and males. | C | 64, 68 |
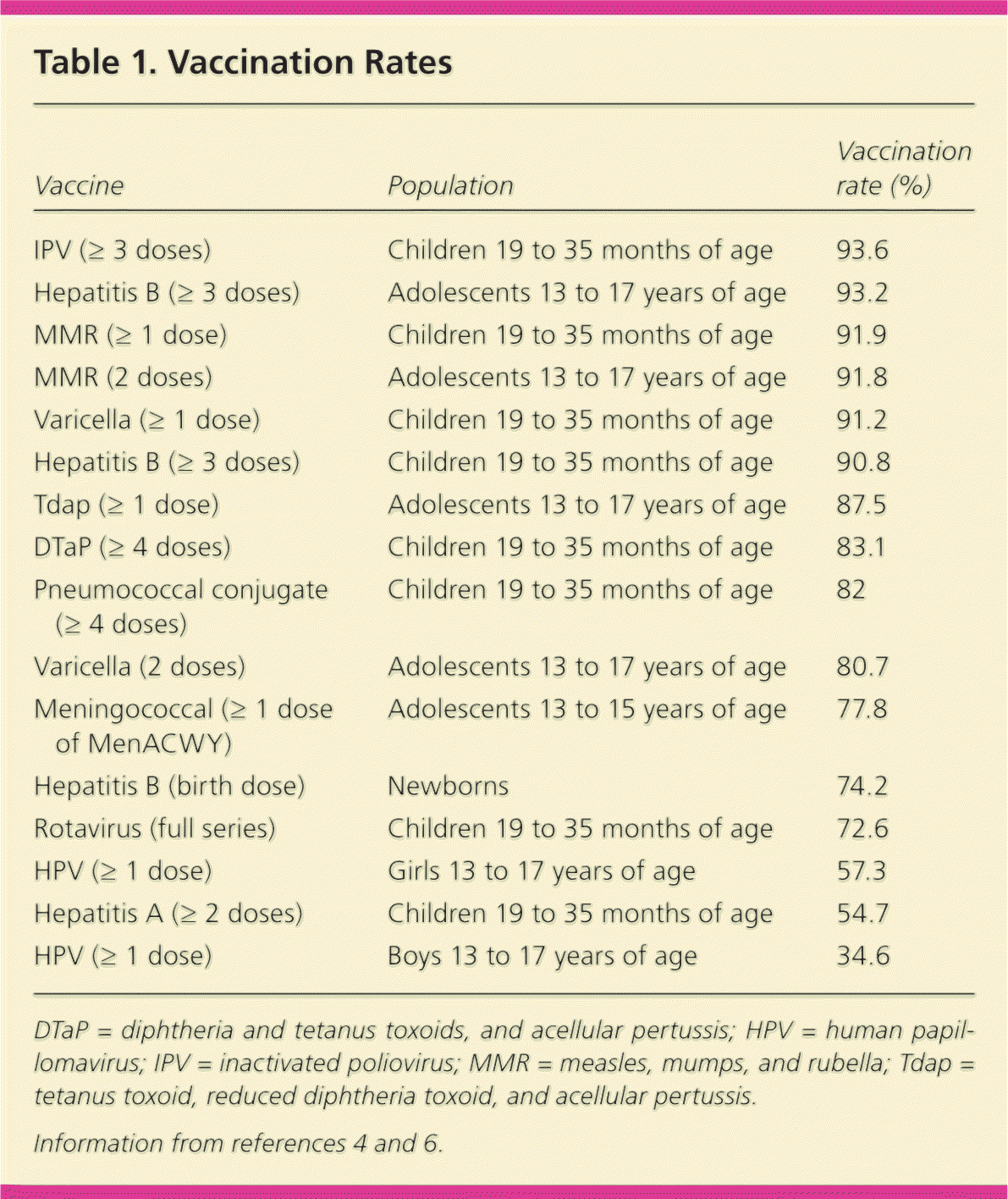
| Vaccine | Population | Vaccination rate (%) |
|---|---|---|
| IPV (≥ 3 doses) | Children 19 to 35 months of age | 93.6 |
| Hepatitis B (≥ 3 doses) | Adolescents 13 to 17 years of age | 93.2 |
| MMR (≥ 1 dose) | Children 19 to 35 months of age | 91.9 |
| MMR (2 doses) | Adolescents 13 to 17 years of age | 91.8 |
| Varicella (≥ 1 dose) | Children 19 to 35 months of age | 91.2 |
| Hepatitis B (≥ 3 doses) | Children 19 to 35 months of age | 90.8 |
| Tdap (≥ 1 dose) | Adolescents 13 to 17 years of age | 87.5 |
| DTaP (≥ 4 doses) | Children 19 to 35 months of age | 83.1 |
| Pneumococcal conjugate (≥ 4 doses) | Children 19 to 35 months of age | 82 |
| Varicella (2 doses) | Adolescents 13 to 17 years of age | 80.7 |
| Meningococcal (≥ 1 dose of MenACWY) | Adolescents 13 to 15 years of age | 77.8 |
| Hepatitis B (birth dose) | Newborns | 74.2 |
| Rotavirus (full series) | Children 19 to 35 months of age | 72.6 |
| HPV (≥ 1 dose) | Girls 13 to 17 years of age | 57.3 |
| Hepatitis A (≥ 2 doses) | Children 19 to 35 months of age | 54.7 |
| HPV (≥ 1 dose) | Boys 13 to 17 years of age | 34.6 |

| Enhancing access to vaccination services (recommended) | |
| Home visits to increase vaccination rates6 | |
| Reducing out-of-pocket costs6 | |
| Vaccination programs for participants in the Special Supplemental Nutrition Program for Women, Infants, and Children6 | |
| Vaccination programs in schools and child care centers6 | |
| Increasing community demand for vaccinations | |
| Insufficient evidence | |
| Clinic-based education when used alone | |
| Community-wide education when used alone | |
| Financial sanctions | |
| Patient-held paper immunization records | |
| Recommended | |
| Community-based interventions implemented in combination | |
| Patient or family reward incentives | |
| Patient reminder and recall systems | |
| Vaccination requirements for child care and school attendance | |
| Physician- or health care system–based interventions | |
| Insufficient evidence | |
| Physician education when used alone | |
| Recommended | |
| Health care system–based interventions implemented in combination | |
| Immunization information systems | |
| Physician assessment and feedback | |
| Physician reminders | |
| Standing orders | |
Reasons for Lack of Immunizations
Low socioeconomic status has been cited as a reason for lack of immunizations. More recently, however, parental reluctance to vaccinate their children has become a growing public health concern.9,10 Reasons for refusing vaccinations include parental concerns that the vaccine has not been on the market long enough and beliefs that their child is at low risk of disease, that they would rather their child get the disease (e.g., varicella), and that the risk of adverse effects is too high. Safety concerns are more prominent with newer vaccines, such as the HPV vaccine. Among adolescents, one of the most important factors in the decision to vaccinate is the physician's recommendation.11–13 Surveys suggest that physicians who graduated more recently believe that children receive too many vaccinations.13 It is unclear whether these beliefs contribute to parental decisions to delay or forgo vaccinations or simply reflect the tendency for parents to choose physicians who have beliefs similar to their own. Although parents place their trust in their child's physician, they also trust non–health professional sources, practitioners of complementary and alternative medicine, social media, and their social networks.14,15 Missed opportunities for vaccination at the adolescent visit are also common; if the HPV vaccine is administered at the same time as another vaccine, the coverage rate for one or more doses could be as high as 92.6%.16
Parental fear of vaccines has resulted in lack of immunization in many communities, leading to recent outbreaks of pertussis.17 From January to May 2014, the Centers for Disease Control and Prevention (CDC) recorded 280 cases of measles, the highest number since the disease was eliminated in 2000. Of these cases, 97% were imported from 18 countries, and 90% of patients were not vaccinated because of philosophical, religious, or personal issues, or had unknown immunization status.18–20 A recent multistate measles outbreak from one source case in California triggered a CDC health advisory in January 2015.21 Although studies have not consistently shown that face-to-face counseling with parents improves acceptance of immunizations, physicians should continue to advocate for immunizations during routine clinical encounters, encouraging the parent's trust, a factor that has been linked to overcoming vaccine hesitancy.22 Resources are available to provide evidence-based education to physicians about vaccines and their effectiveness, as well as to reassure parents that vaccines are safe and effective.23 Forms are also available to document parents' refusal to vaccinate.24,25
Vaccines and Schedules for Children
The 2015 schedules of recommended vaccinations for children and adolescents are available at http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Some recommendations for routine vaccinations for healthy children (e.g., hepatitis A and B; diphtheria and tetanus toxoids, and acellular pertussis [DTaP]; Hib; inactivated poliovirus [IPV]; measles, mumps, and rubella; varicella) have not changed significantly. For other routine vaccinations, there have been significant changes in recent years (Table 3).1,26,27 Data obtained by the CDC through the Vaccine Adverse Event Reporting System, Vaccine Safety Datalink, and Clinical Immunization Safety Assessment provide physicians with evidence that currently approved vaccines are safe and effective (Table 4).28–43
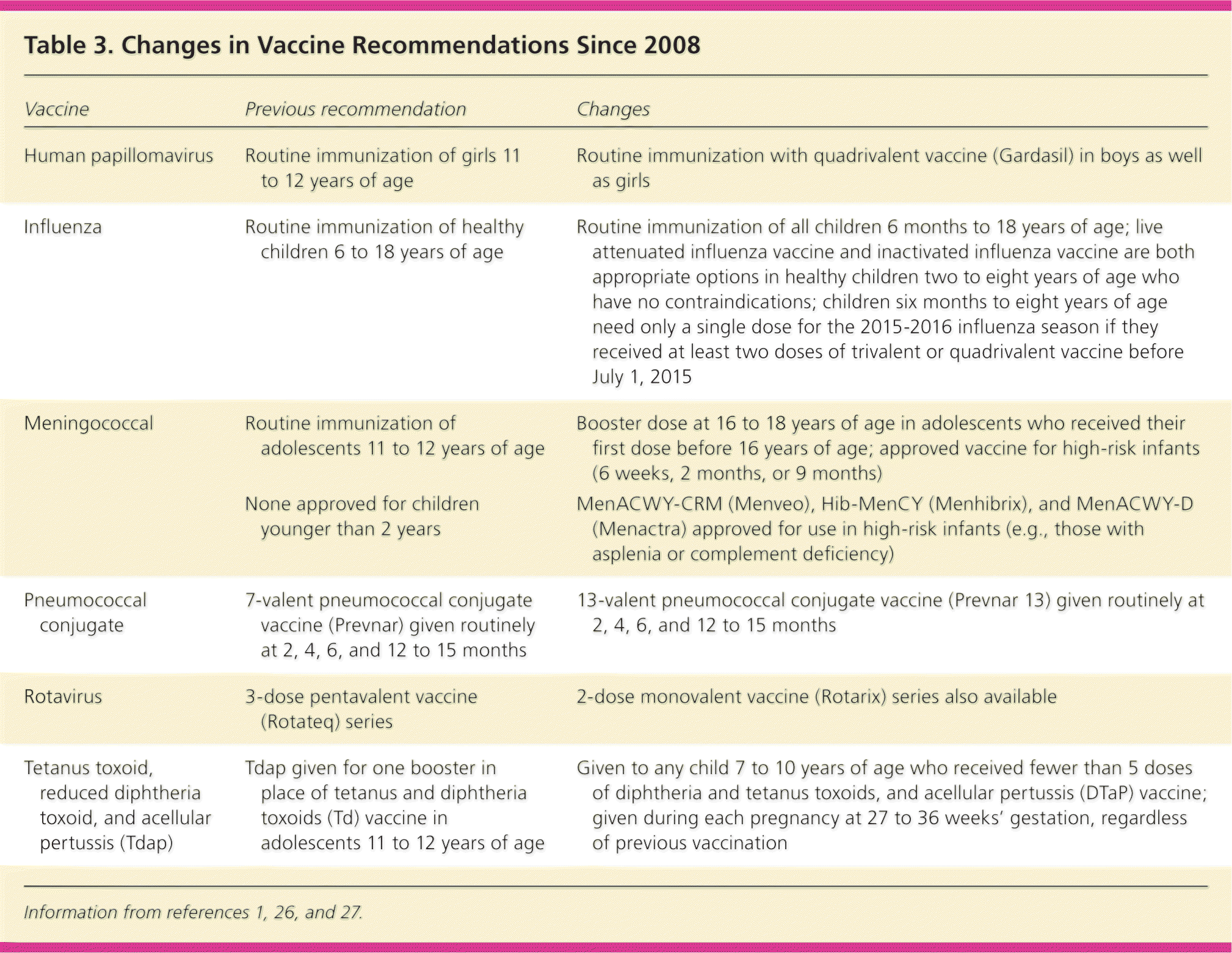
| Vaccine | Previous recommendation | Changes |
|---|---|---|
| Human papillomavirus | Routine immunization of girls 11 to 12 years of age | Routine immunization with quadrivalent vaccine (Gardasil) in boys as well as girls |
| Influenza | Routine immunization of healthy children 6 to 18 years of age | Routine immunization of all children 6 months to 18 years of age; live attenuated influenza vaccine and inactivated influenza vaccine are both appropriate options in healthy children two to eight years of age who have no contraindications; children six months to eight years of age need only a single dose for the 2015–2016 influenza season if they received at least two doses of trivalent or quadrivalent vaccine before July 1, 2015 |
| Meningococcal | Routine immunization of adolescents 11 to 12 years of age | Booster dose at 16 to 18 years of age in adolescents who received their first dose before 16 years of age; approved vaccine for high-risk infants (6 weeks, 2 months, or 9 months) |
| None approved for children younger than 2 years | MenACWY-CRM (Menveo), Hib-MenCY (Menhibrix), and MenACWY-D (Menactra) approved for use in high-risk infants (e.g., those with asplenia or complement deficiency) | |
| Pneumococcal conjugate | 7-valent pneumococcal conjugate vaccine (Prevnar) given routinely at 2, 4, 6, and 12 to 15 months | 13-valent pneumococcal conjugate vaccine (Prevnar 13) given routinely at 2, 4, 6, and 12 to 15 months |
| Rotavirus | 3-dose pentavalent vaccine (Rotateq) series | 2-dose monovalent vaccine (Rotarix) series also available |
| Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) | Tdap given for one booster in place of tetanus and diphtheria toxoids (Td) vaccine in adolescents 11 to 12 years of age | Given to any child 7 to 10 years of age who received fewer than 5 doses of diphtheria and tetanus toxoids, and acellular pertussis (DTaP) vaccine; given during each pregnancy at 27 to 36 weeks' gestation, regardless of previous vaccination |
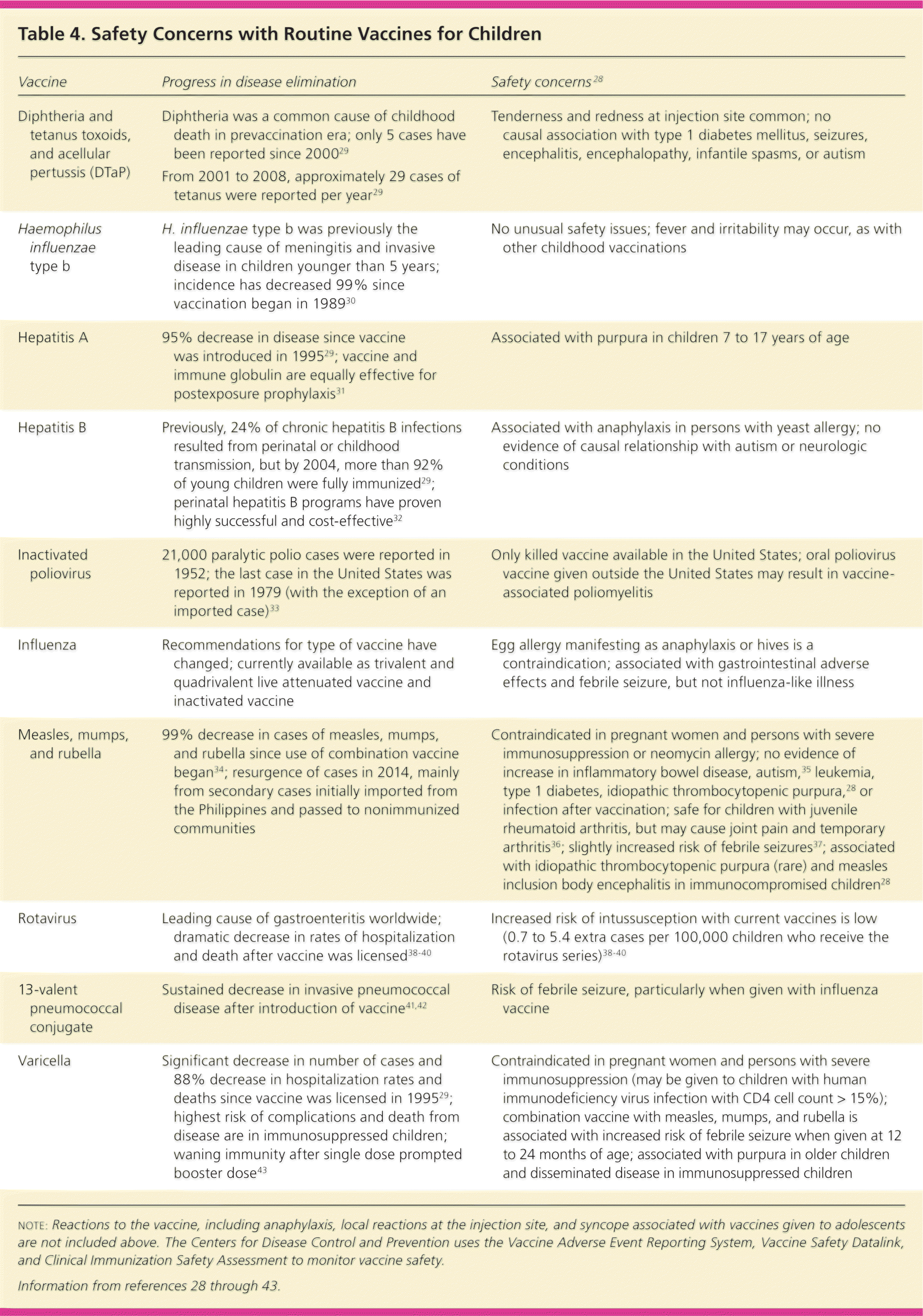
| Vaccine | Progress in disease elimination | Safety concerns28 |
|---|---|---|
| Diphtheria and tetanus toxoids, and acellular pertussis (DTaP) | Tenderness and redness at injection site common; no causal association with type 1 diabetes mellitus, seizures, encephalitis, encephalopathy, infantile spasms, or autism | |
| Haemophilus influenzae type b | H. influenzae type b was previously the leading cause of meningitis and invasive disease in children younger than 5 years; incidence has decreased 99% since vaccination began in 198930 | No unusual safety issues; fever and irritability may occur, as with other childhood vaccinations |
| Hepatitis A | 95% decrease in disease since vaccine was introduced in 199529; vaccine and immune globulin are equally effective for postexposure prophylaxis31 | Associated with purpura in children 7 to 17 years of age |
| Hepatitis B | Previously, 24% of chronic hepatitis B infections resulted from perinatal or childhood transmission, but by 2004, more than 92% of young children were fully immunized29; perinatal hepatitis B programs have proven highly successful and cost-effective32 | Associated with anaphylaxis in persons with yeast allergy; no evidence of causal relationship with autism or neurologic conditions |
| Inactivated poliovirus | 21,000 paralytic polio cases were reported in 1952; the last case in the United States was reported in 1979 (with the exception of an imported case)33 | Only killed vaccine available in the United States; oral poliovirus vaccine given outside the United States may result in vaccine-associated poliomyelitis |
| Influenza | Recommendations for type of vaccine have changed; currently available as trivalent and quadrivalent live attenuated vaccine and inactivated vaccine | Egg allergy manifesting as anaphylaxis or hives is a contraindication; associated with gastrointestinal adverse effects and febrile seizure, but not influenza-like illness |
| Measles, mumps, and rubella | 99% decrease in cases of measles, mumps, and rubella since use of combination vaccine began34; resurgence of cases in 2014, mainly from secondary cases initially imported from the Philippines and passed to nonimmunized communities | Contraindicated in pregnant women and persons with severe immunosuppression or neomycin allergy; no evidence of increase in inflammatory bowel disease, autism,35 leukemia, type 1 diabetes, idiopathic thrombocytopenic purpura,28 or infection after vaccination; safe for children with juvenile rheumatoid arthritis, but may cause joint pain and temporary arthritis36; slightly increased risk of febrile seizures37; associated with idiopathic thrombocytopenic purpura (rare) and measles inclusion body encephalitis in immunocompromised children28 |
| Rotavirus | Leading cause of gastroenteritis worldwide; dramatic decrease in rates of hospitalization and death after vaccine was licensed38–40 | Increased risk of intussusception with current vaccines is low (0.7 to 5.4 extra cases per 100,000 children who receive the rotavirus series)38–40 |
| 13-valent pneumococcal conjugate | Sustained decrease in invasive pneumococcal disease after introduction of vaccine41,42 | Risk of febrile seizure, particularly when given with influenza vaccine |
| Varicella | Significant decrease in number of cases and 88% decrease in hospitalization rates and deaths since vaccine was licensed in 199529; highest risk of complications and death from disease are in immunosuppressed children; waning immunity after single dose prompted booster dose43 | Contraindicated in pregnant women and persons with severe immunosuppression (may be given to children with human immunodeficiency virus infection with CD4 cell count > 15%); combination vaccine with measles, mumps, and rubella is associated with increased risk of febrile seizure when given at 12 to 24 months of age; associated with purpura in older children and disseminated disease in immunosuppressed children |
HIB VACCINES
Two Hib vaccines, PRP-T (ActHIB, Hiberix) and PRP-OMP (PedvaxHIB), have been approved by the U.S. Food and Drug Administration since 1987. Data indicate that PRP-OMP elicits a stronger immune response with the first injection, and it is recommended for use in American Indians and Alaska Natives, who have 8% to 10% higher disease rates, as well as for children with functional asplenia or immunosuppression30 (eTable A). Combined vaccines (e.g., DTaP/IPV/PRP-T [Pentacel], PRP-OMP/hepatitis B [Comvax]) have equal immunogenicity to individual vaccines and are often more acceptable to parents.44
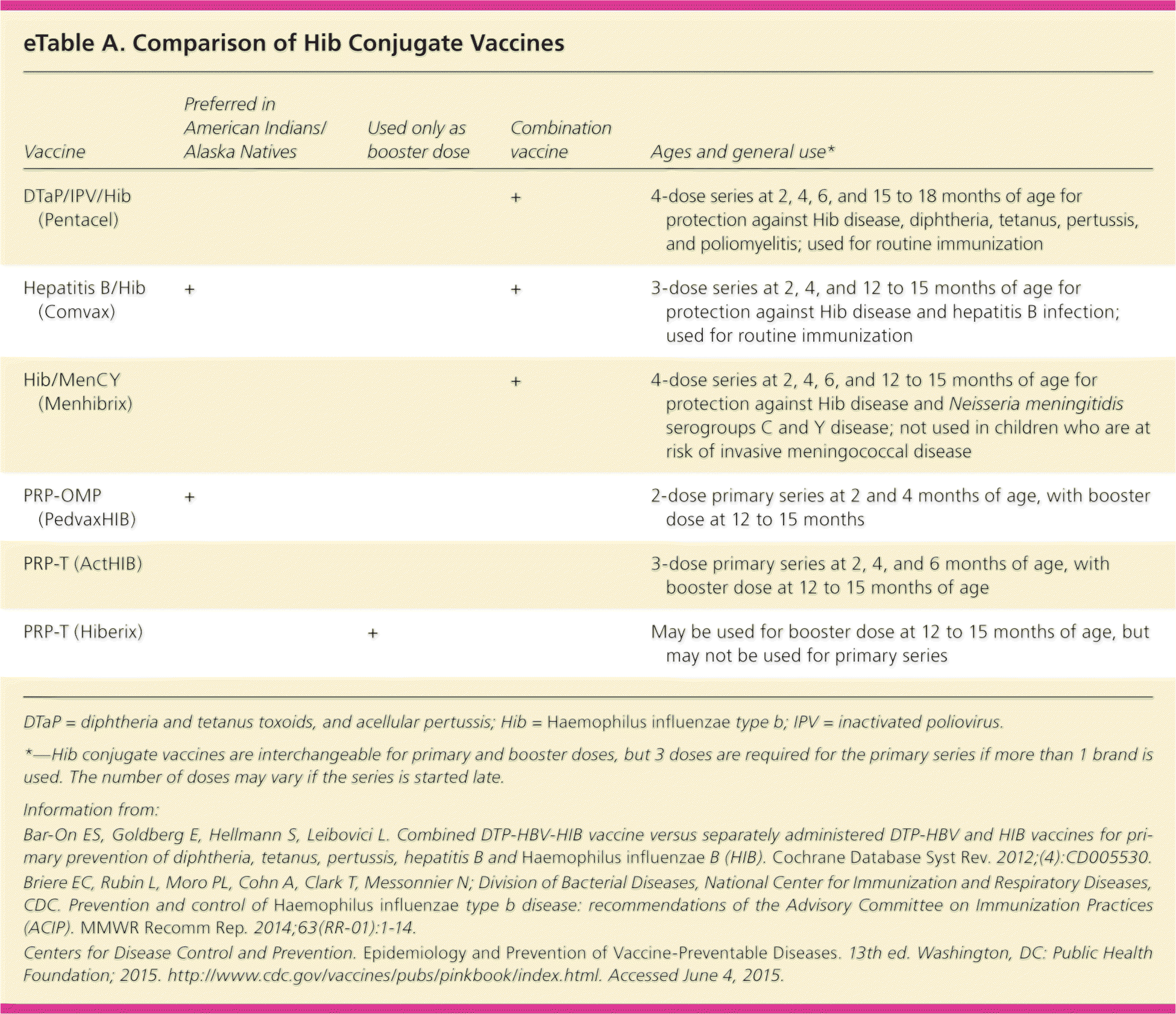
| Vaccine | Preferred in American Indians/Alaska Natives | Used only as booster dose | Combination vaccine | Ages and general use* |
|---|---|---|---|---|
| DTaP/IPV/Hib (Pentacel) | + | 4-dose series at 2, 4, 6, and 15 to 18 months of age for protection against Hib disease, diphtheria, tetanus, pertussis, and poliomyelitis; used for routine immunization | ||
| Hepatitis B/Hib (Comvax) | + | + | 3-dose series at 2, 4, and 12 to 15 months of age for protection against Hib disease and hepatitis B infection; used for routine immunization | |
| Hib/MenCY (Menhibrix) | + | 4-dose series at 2, 4, 6, and 12 to 15 months of age for protection against Hib disease and Neisseria meningitidis serogroups C and Y disease; not used in children who are at risk of invasive meningococcal disease | ||
| PRP-OMP (PedvaxHIB) | + | 2-dose primary series at 2 and 4 months of age, with booster dose at 12 to 15 months | ||
| PRP-T (ActHIB) | 3-dose primary series at 2, 4, and 6 months of age, with booster dose at 12 to 15 months of age | |||
| PRP-T (Hiberix) | + | May be used for booster dose at 12 to 15 months of age, but may not be used for primary series |
PNEUMOCOCCAL VACCINES
Since 2000, when the first pneumococcal conjugate vaccine (7-valent pneumococcal conjugate vaccine [PCV7; Prevnar]) was introduced, rates of invasive pneumococcal disease and pneumonia have dramatically decreased. The newer PCV13 vaccine (Prevnar 13), which replaced PCV7 in the routine immunization schedule in 2011, expands coverage of a disease that continues to cause significant morbidity and mortality.41,45 The 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23) is recommended routinely for only high-risk children two years and older. However, corticosteroid-dependent asthma is now considered a chronic lung condition for which PPSV23 vaccine is recommended42 (eTable B).
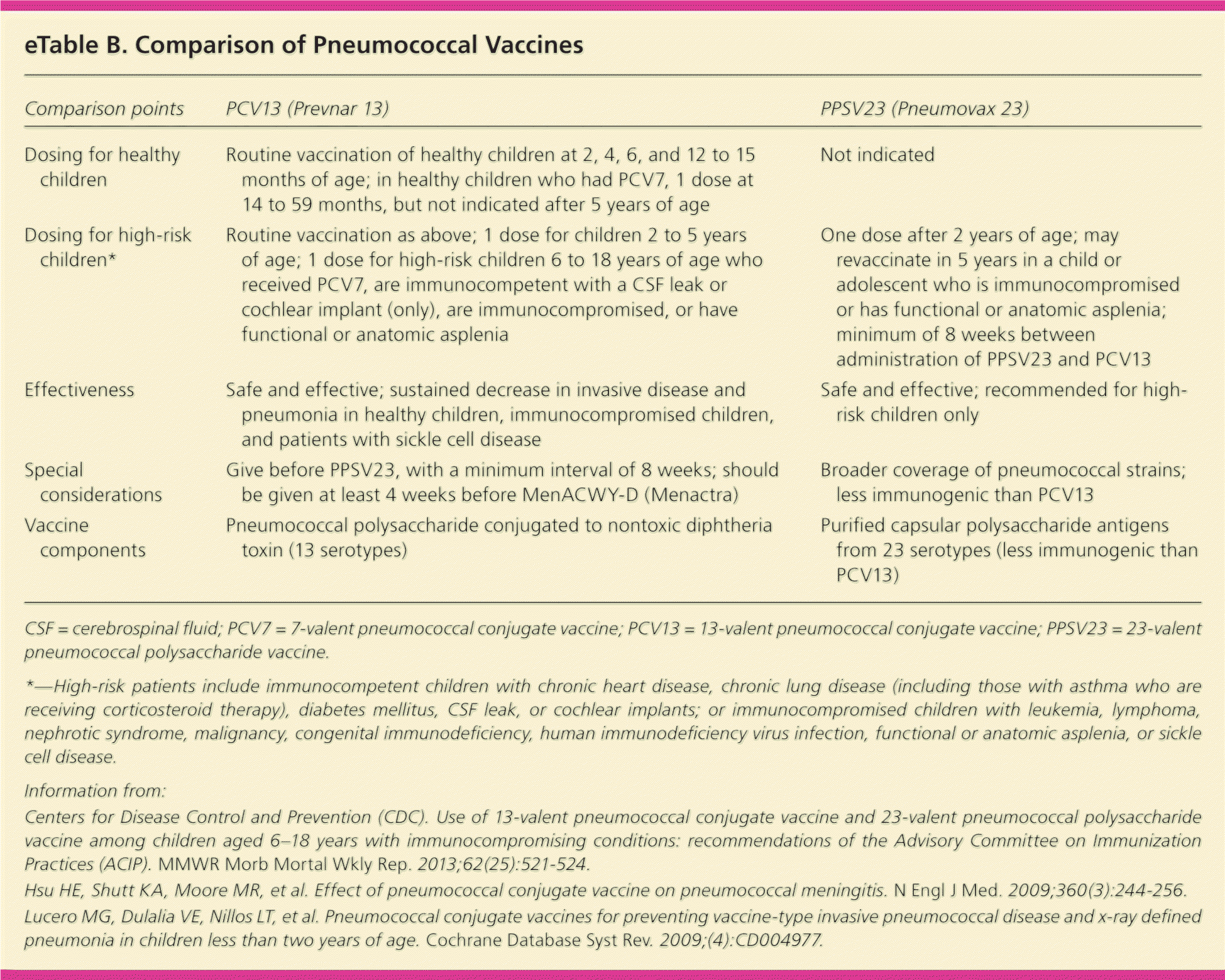
| Comparison points | PCV13 (Prevnar 13) | PPSV23 (Pneumovax 23) |
|---|---|---|
| Dosing for healthy children | Routine vaccination of healthy children at 2, 4, 6, and 12 to 15 months of age; in healthy children who had PCV7, 1 dose at 14 to 59 months, but not indicated after 5 years of age | Not indicated |
| Dosing for high-risk children* | Routine vaccination as above; 1 dose for children 2 to 5 years of age; 1 dose for high-risk children 6 to 18 years of age who received PCV7, are immunocompetent with a CSF leak or cochlear implant (only), are immunocompromised, or have functional or anatomic asplenia | One dose after 2 years of age; may revaccinate in 5 years in a child or adolescent who is immunocompromised or has functional or anatomic asplenia; minimum of 8 weeks between administration of PPSV23 and PCV13 |
| Effectiveness | Safe and effective; sustained decrease in invasive disease and pneumonia in healthy children, immunocompromised children, and patients with sickle cell disease | Safe and effective; recommended for high-risk children only |
| Special considerations | Give before PPSV23, with a minimum interval of 8 weeks; should be given at least 4 weeks before MenACWY-D (Menactra) | Broader coverage of pneumococcal strains; less immunogenic than PCV13 |
| Vaccine components | Pneumococcal polysaccharide conjugated to nontoxic diphtheria toxin (13 serotypes) | Purified capsular polysaccharide antigens from 23 serotypes (less immunogenic than PCV13) |
ROTAVIRUS VACCINES
Rotavirus vaccination is effective in reducing severe diarrheal illness. It reduced cases of severe rotavirus diarrhea by more than 80% in children younger than two years in low-mortality countries, and by 40% to 57% in those in high-mortality countries.38 The vaccine is now available as a monovalent two-dose series (RV1; Rotarix) or pentavalent three-dose series (RV5; Rotateq).1 These formulations are equally effective and cost-effective, and both result in a minimally increased risk of intussusception on days 3 to 6 after the first dose.39 The risk of intussusception (0.79 in 100,000 cases) is minimal compared with the 40,000 fewer hospitalizations for diarrheal illness.39 Although RV5 covers more strains of rotavirus, RV1 requires only two doses and thus has higher compliance rates.40
INFLUENZA VACCINES
Influenza is a major cause of morbidity and mortality in the United States. Since the 2003–2004 season when the CDC began tracking influenza deaths in children, the highest number occurred during the 2009–2010 pandemic, with 358 deaths reported.46 In 2010, the CDC's Advisory Committee on Immunization Practices (ACIP) first recommended annual influenza vaccination for all children and adolescents older than six months.46–48 An analysis of 830 influenza-associated deaths in children from 2004 to 2012 showed that 43% had no high-risk medical condition, and that these children were more likely to die before hospital admission.49 During the 2014–2015 influenza season, 141 laboratory-confirmed influenza deaths in children had been reported in 40 states as of May 23, predominantly from influenza A (H3N2). The vaccine during this season offered reduced protection against the circulating strains, with an estimated 18% to 19% effectiveness.50
The virus composition of the 2014–2015 vaccine was unchanged from 2013–2014. However, the vaccine for 2015–2016 represents a change in the influenza A (H3N2) and influenza B viruses. Children six months to eight years of age who received at least two doses of trivalent or quadrivalent influenza vaccine before July 1, 2015, need only a single dose for the 2015–2016 influenza season.27 Those who received only one dose before July 1, 2015, or who have not been vaccinated previously should receive two doses, at least four weeks apart, for the 2015-2016 season. [ corrected] The Fluzone trivalent and quadrivalent inactivated influenza vaccines are approved for children six months and older, and the Fluarix quadrivalent inactivated vaccine is approved for persons three years and older.27
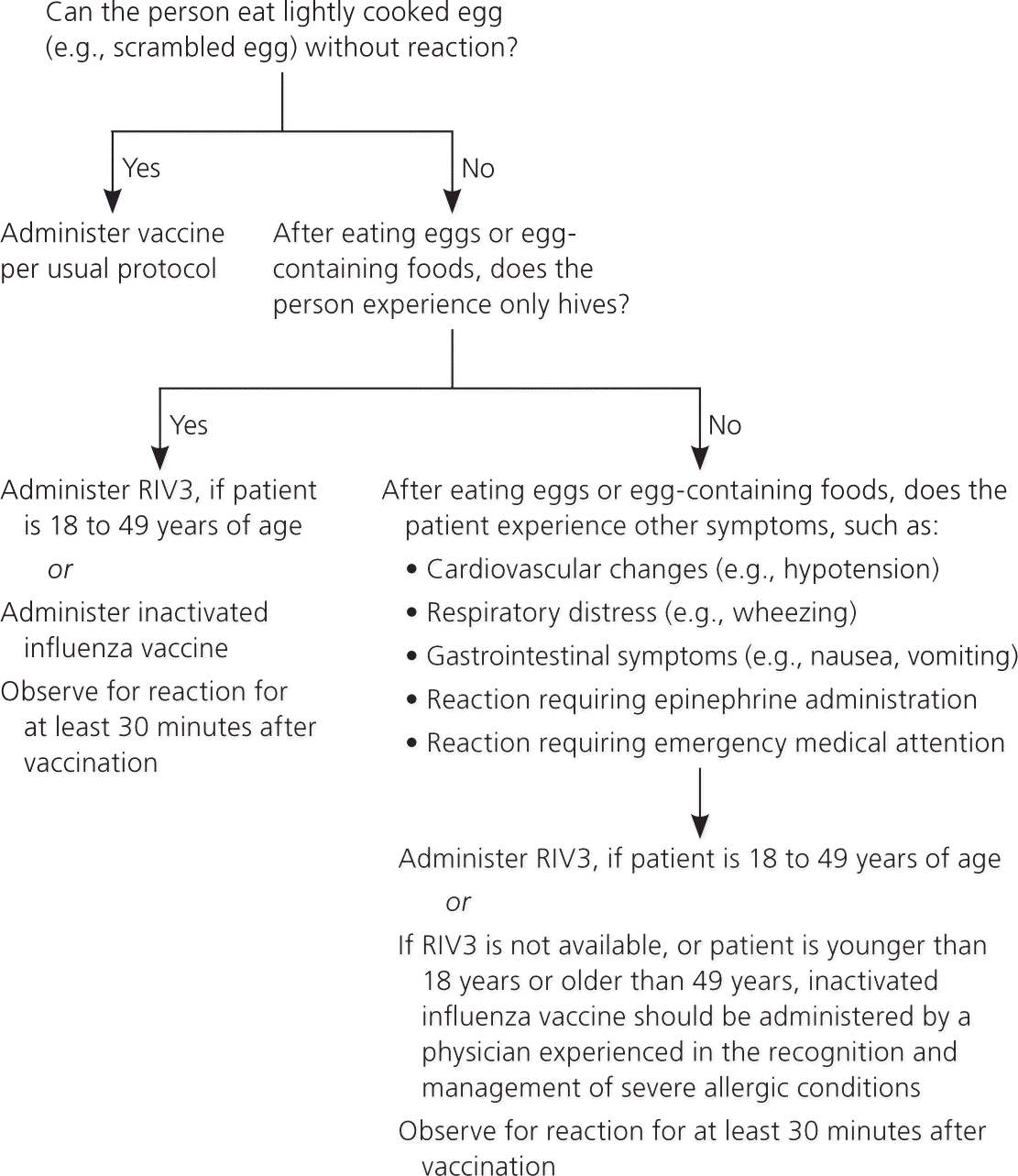
The live attenuated influenza vaccine (LAIV), Flumist, is available only as a quadrivalent vaccine and is approved for persons two to 49 years of age.47,48 A 2012 Cochrane review found that LAIV is more effective in children six years and younger51; however, research with more recent influenza strains suggests that there is no difference in effectiveness between LAIV and inactivated vaccines. ACIP previously recommended LAIV over inactivated vaccine in children two to eight years of age; however, based on the absence of consistent data showing greater effectiveness of LAIV, it is no longer preferred over inactivated vaccine.27 eTable C lists precautions and contraindications for the use of LAIV. The two newest influenza vaccines, Flublok and Flucelvax, are preferred in persons with egg allergy, but they have not been approved for children; children with milder allergies and those who can eat lightly cooked eggs can usually receive inactivated vaccine (Figure 1).48 The recombinant, cell-based, and intradermal vaccines are approved for persons 18 years and older; high-dose inactivated vaccine is approved for those 65 years and older.48 Although no influenza vaccine is available for children younger than six months, there is some evidence that immunization of pregnant women protects newborns and decreases hospitalization of infants younger than six months.52

| Contraindications |
| Asthma |
| Children and adolescents receiving concomitant aspirin therapy |
| Children younger than 2 years and adults older than 50 years |
| Children 2 to 4 years of age who have had wheezing or asthma in the past year |
| Chronic lung, cardiac, renal, liver, neurologic, hematologic, or metabolic disorders |
| Egg allergy with anaphylaxis or severe hives |
| History of severe hypersensitivity reaction to any component of the vaccine or to a previous dose of any influenza vaccine |
| Immunosuppressed children or adults |
| Pregnancy |
| Precautions |
| Egg allergy (Figure 1) |
| Guillain-Barré syndrome within 6 weeks of a previous dose of influenza vaccine |
| Moderate or severe acute illness, with or without fever |
| Severe congestion |
DIPHTHERIA, TETANUS, AND PERTUSSIS VACCINES
Recommendations to vaccinate children with DTaP have not changed; a five-dose series is still given at two months, four months, six months, 12 to 15 months, and four to six years of age (eTable D). In 1997, when the whole-cell DTP series was replaced by acellular DTaP, pertussis immunity began to wane, and unprecedented changes in the epidemiology of the disease began. By 2000, the rate reported in adolescents 11 to 19 years of age exceeded that for younger children, even among those who were fully vaccinated.53,54 A decrease in vaccine effectiveness (from 95% to 71%) occurred in the five years after the fifth dose.55 Global concern over this decrease led the World Health Organization to recommend that countries with fewer than five doses in the primary series continue to use whole-cell pertussis vaccines.56

| Vaccine | Ages and general use |
|---|---|
| DT | 6 weeks to 6 years |
| Not used routinely; use in patients allergic to pertussis component | |
| DTaP (Daptacel, Infanrix) | 6 weeks to 6 years (Tdap given after 7 years) |
| 5-dose series (2, 4, 6, and 12 to 15 months, and 4 to 6 years) unless fourth dose was given at or after 4 years of age; Daptacel not licensed for fifth dose | |
| DTaP/hepatitis B/IPV (Pediarix) | 6 weeks to 6 years |
| Primary immunization series (3 doses at 2, 4, and 6 months); not licensed for booster dose or birth dose of hepatitis B vaccine | |
| DTaP/IPV (Kinrix) | 4 to 6 years |
| Approved only for booster dose and only for fourth dose of IPV | |
| DTaP/IPV/PRP-T (Pentacel) | 6 weeks to 4 years |
| 2, 4, 6, and 12 to 15 months doses only | |
| Td | Older than 7 years |
| Not routinely given to children; used for booster or catch-up doses if children received Tdap early | |
| Tdap (Boostrix, Adacel) | Routinely given to adolescents, and thereafter is given during each pregnancy between 27 and 36 weeks' gestation; fifth dose if child had fewer than 5 doses of DTaP; given once to adults caring for young children |
Currently, the highest rate of pertussis (160 per 100,000 persons) occurs in infants younger than two months, and more than 80% of these infants are hospitalized.57 Consequently, ACIP replaced the tetanus and diphtheria toxoids (Td) booster with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap)53 for adolescents 11 to 18 years of age and for adults, then recommended booster doses for child-care workers, health professionals, parents, grandparents older than 65 years, children seven to 10 years of age who did not receive five doses in the primary series, and, most recently, with each pregnancy (at 27 to 36 weeks' gestation)58 to reduce the exposure of infants to pertussis.59
MENINGOCOCCAL VACCINES
Disease caused by Neisseria meningitidis develops rapidly, even among healthy children and adolescents, resulting in high morbidity and mortality. There are now fewer than 1,000 cases of meningococcal disease per year in the United States, and rates have been declining since the late 1990s, making it difficult for randomized controlled trials to evaluate the effectiveness of meningococcal vaccines.60,61
Serogroups B, C, and Y each account for approximately one-third of cases in the United States, although the proportion of cases caused by each serogroup varies with age; serogroups A and W circulate globally.60 Type B causes most cases of meningococcal disease in infants; the highest rates are in children younger than one year. Three vaccines are approved for young children who are at increased risk, but they do not provide protection against serotype B and thus are not routinely recommended in children who do not have risk factors60–63 (eTable E).
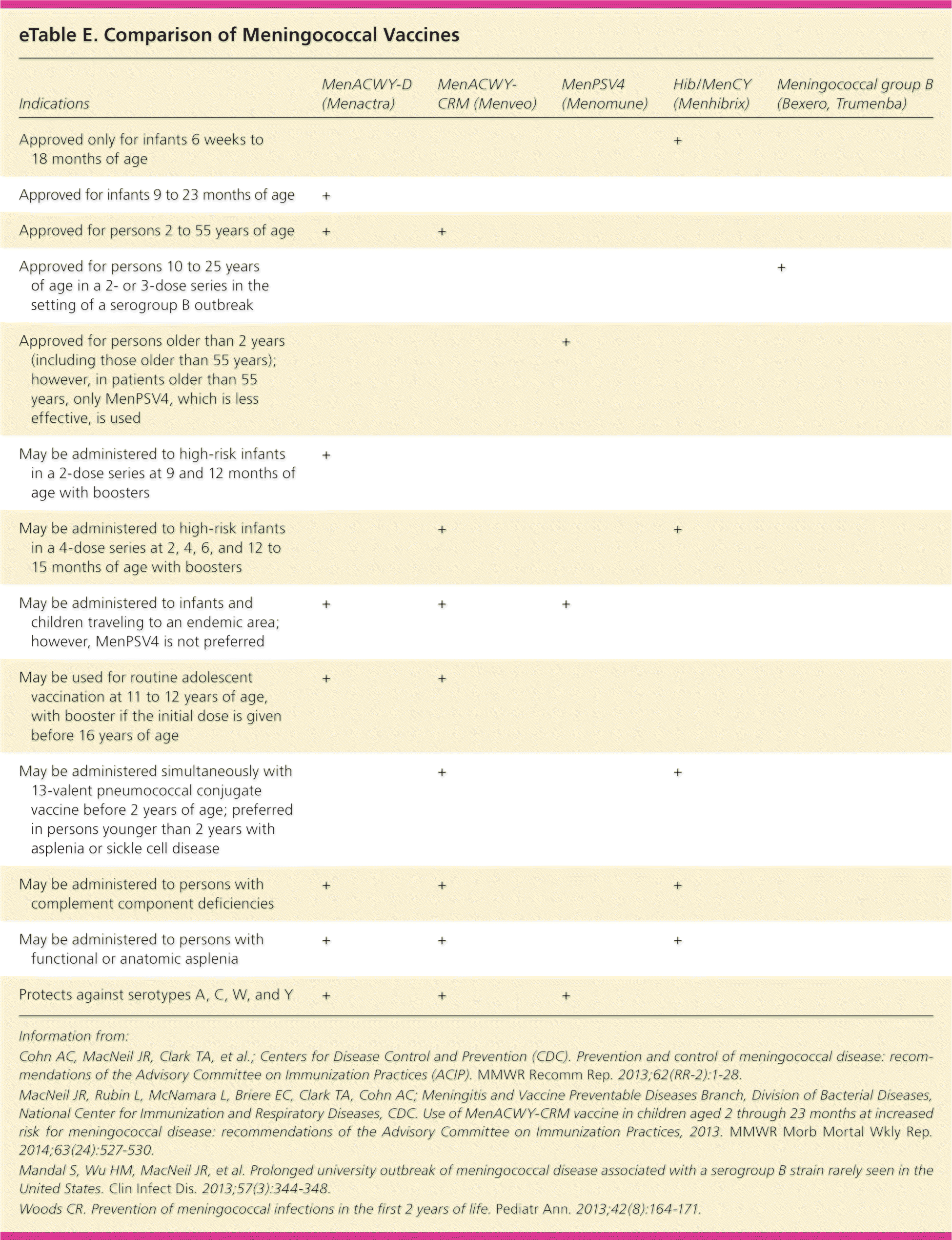
| Indications | MenACWY-D (Menactra) | MenACWY-CRM (Menveo) | MenPSV4 (Menomune) | Hib/MenCY (Menhibrix) | Meningococcal group B (Bexero, Trumenba) |
|---|---|---|---|---|---|
| Approved only for infants 6 weeks to 18 months of age | + | ||||
| Approved for infants 9 to 23 months of age | + | ||||
| Approved for persons 2 to 55 years of age | + | + | |||
| Approved for persons 10 to 25 years of age in a 2- or 3-dose series in the setting of a serogroup B outbreak | + | ||||
| Approved for persons older than 2 years (including those older than 55 years); however, in patients older than 55 years, only MenPSV4, which is less effective, is used | + | ||||
| May be administered to high-risk infants in a 2-dose series at 9 and 12 months of age with boosters | + | ||||
| May be administered to high-risk infants in a 4-dose series at 2, 4, 6, and 12 to 15 months of age with boosters | + | + | |||
| May be administered to infants and children traveling to an endemic area; however, MenPSV4 is not preferred | + | + | + | ||
| May be used for routine adolescent vaccination at 11 to 12 years of age, with booster if the initial dose is given before 16 years of age | + | + | |||
| May be administered simultaneously with 13-valent pneumococcal conjugate vaccine before 2 years of age; preferred in persons younger than 2 years with asplenia or sickle cell disease | + | + | |||
| May be administered to persons with complement component deficiencies | + | + | + | ||
| May be administered to persons with functional or anatomic asplenia | + | + | + | ||
| Protects against serotypes A, C, W, and Y | + | + | + |
The second peak of disease occurs in adolescence, with types C, Y, and W causing 73% of cases among persons 11 years and older.60 ACIP recommends routine meningococcal vaccination in adolescents 11 to 18 years of age.60 Both quadrivalent meningococcal polysaccharide-protein conjugate vaccines approved for adolescents (MenACWY-D [Menactra] and MenACWY-CRM [Menveo]) provide protection against serotypes A, C, W, and Y. Because of demonstrated loss of immunity over time (82% one year postvaccination to 59% at three to six years), ACIP recommends a booster dose at 16 years of age, and for those at continued risk.61,62 Although colleges in 36 states require meningococcal vaccination, 98% of cases are sporadic, and overall incidence among college students is similar to or lower than that in the general population; recent outbreaks on college campuses have also been caused by type B, necessitating the use of a vaccine initially approved outside the United States.63
In October 2014, the first serogroup B vaccine (Trumenba) was approved by the U.S. Food and Drug Administration as a three-dose series for persons 10 to 25 years of age, followed by a second vaccine (Bexero), which is given as a two-dose series. These vaccines are recommended only in the setting of a serotype B outbreak. The initial precaution for persons with a history of Guillain-Barré syndrome was removed after post-licensure surveillance studies were performed.60
HPV VACCINES
Genital HPV is the most common sexually transmitted infection in the United States. Of the more than 150 HPV types, approximately 40 are linked with genital HPV infection and are classified as high or low risk according to their epidemiologic association with cervical cancer. Although most HPV infections are transient, persistent infection may result in genital warts, cervical dysplasia, and cervical, anogenital, and oropharnygeal cancers.64
Two HPV vaccines are recommended for routine immunization of adolescents 11 to 12 years of age: a bivalent vaccine (Cervarix) for girls and a quadrivalent vaccine (Gardasil) for both boys and girls. Both vaccines provide protection against high-risk HPV types 16 and 18, which cause 70% of cervical cancers, but only the quadrivalent vaccine protects against HPV types 6 and 11, which cause more than 90% of genital warts and recurrent respiratory papillomatosis.64 Both vaccines are administered as a three-shot series given at 0, one to two, and six months64 (eTable F). Some evidence suggests that using two shots instead of three is effective and can result in cost savings if immunogenicity lasts for 20 years65,66; however, immunogenicity data are currently available only for eight years.67
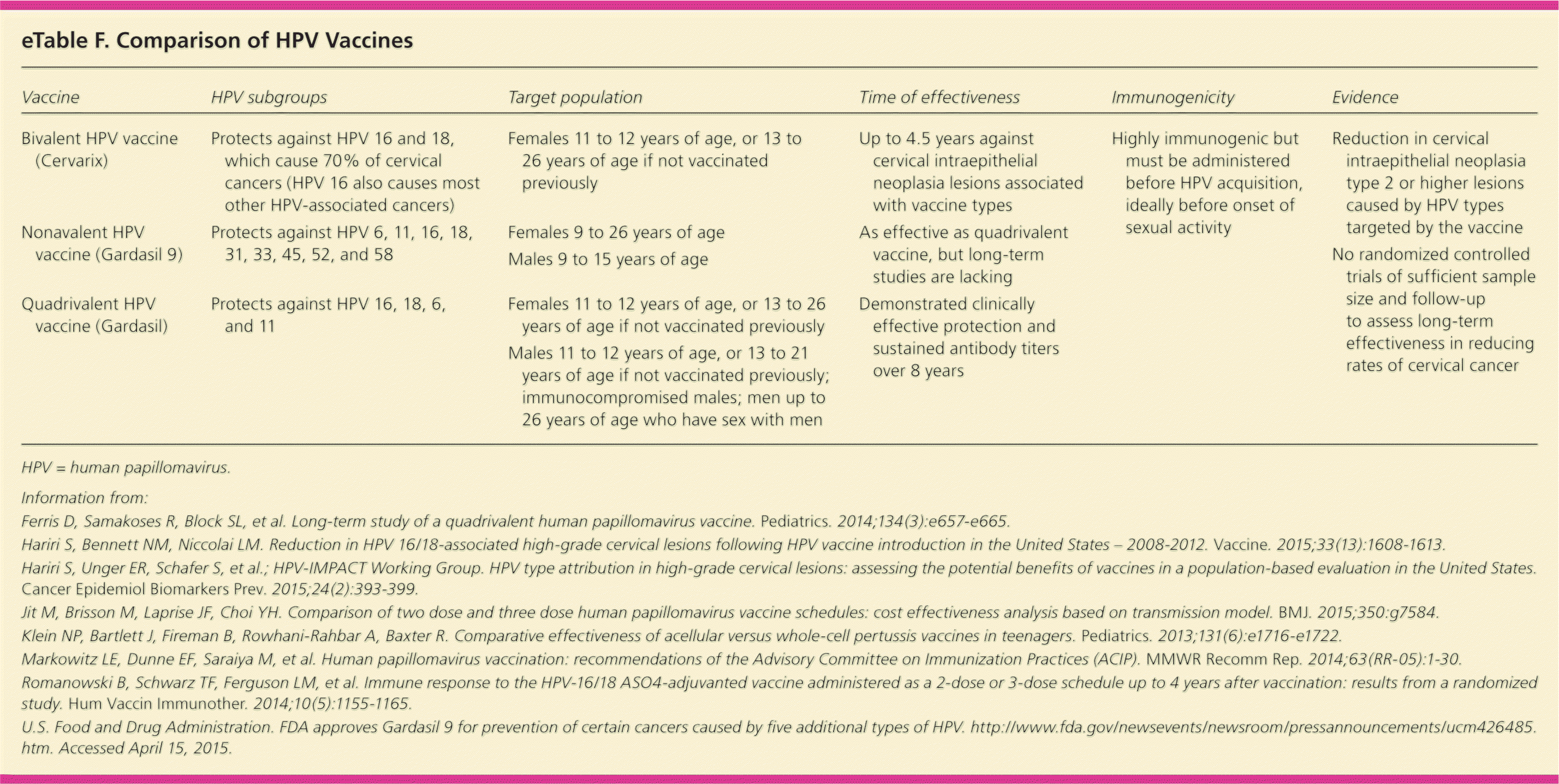
| Vaccine | HPV subgroups | Target population | Time of effectiveness | Immunogenicity | Evidence |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
| ||
|
|
|
|
Overall, 50% of high-grade cervical lesions (cervical intraepithelial neoplasia grade 2 or higher) were attributable to HPV types 16 and 18, and 25% to types 31, 33, 45, 52, and 58.68 Population-based studies have shown a reduction in the prevalence of HPV types 16 and 18–induced high-grade cervical lesions (from 53.6% to 28.4%).69 In December 2014, a nonavalent HPV vaccine effective against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 (Gardasil 9) was approved for females nine to 26 years of age and males nine to 15 years of age. It is estimated to protect against approximately 90% of HPV-related cervical, vulvar, vaginal, and anal cancers.70 The three-dose series costs approximately $1,100.71
Data Sources: The authors reviewed Essential Evidence Plus, and a PubMed search was completed in Clinical Queries using the key terms childhood immunization, adolescent immunization, lack of immunization, efficacy, immunogenicity, safety, combined vaccines, and the name of each vaccine and associated disease. The Cochrane database, Clinical Evidence, Agency for Healthcare Research and Quality evidence reports, and National Guideline Clearinghouse database were also searched. Search dates: July 3, August 10, and September 12, 2014, and June 6, 2015.
