Am Fam Physician. 2015;92(7):588-595
Related editorial: Pharmacogenetic Testing--An Unfulfilled Promise.
Author disclosure: No relevant financial affiliations.
Clinical pharmacogenetics, the use of genetic data to guide drug therapy decisions, is beginning to be used for medications commonly prescribed by family physicians. However, clinicians are largely unfamiliar with principles supporting clinical use of this type of data. For example, genetic variability in the cytochrome P450 2D6 drug metabolizing enzyme can alter the clinical effects of some opioid analgesics (e.g., codeine, tramadol), whereas variability in the CYP2C19 enzyme affects the antiplatelet agent clopidogrel. If testing is performed, patients who are ultrarapid or poor metabolizers of CYP2D6 should avoid codeine use (and possibly tramadol, hydrocodone, and oxycodone) because of the potential for increased toxicity or lack of effectiveness. Patients undergoing percutaneous coronary intervention for acute coronary syndromes who are known to be poor metabolizers of CYP2C19 should consider alternate antiplatelet therapy (e.g., ticagrelor, prasugrel). Some guidelines are available that address appropriate drug therapy changes, and others are in development. Additionally, a number of clinical resources are emerging to support family physicians in the use of pharmacogenetics. When used appropriately, pharmacogenetic testing can be a practical tool to optimize drug therapy and avoid medication adverse effects.
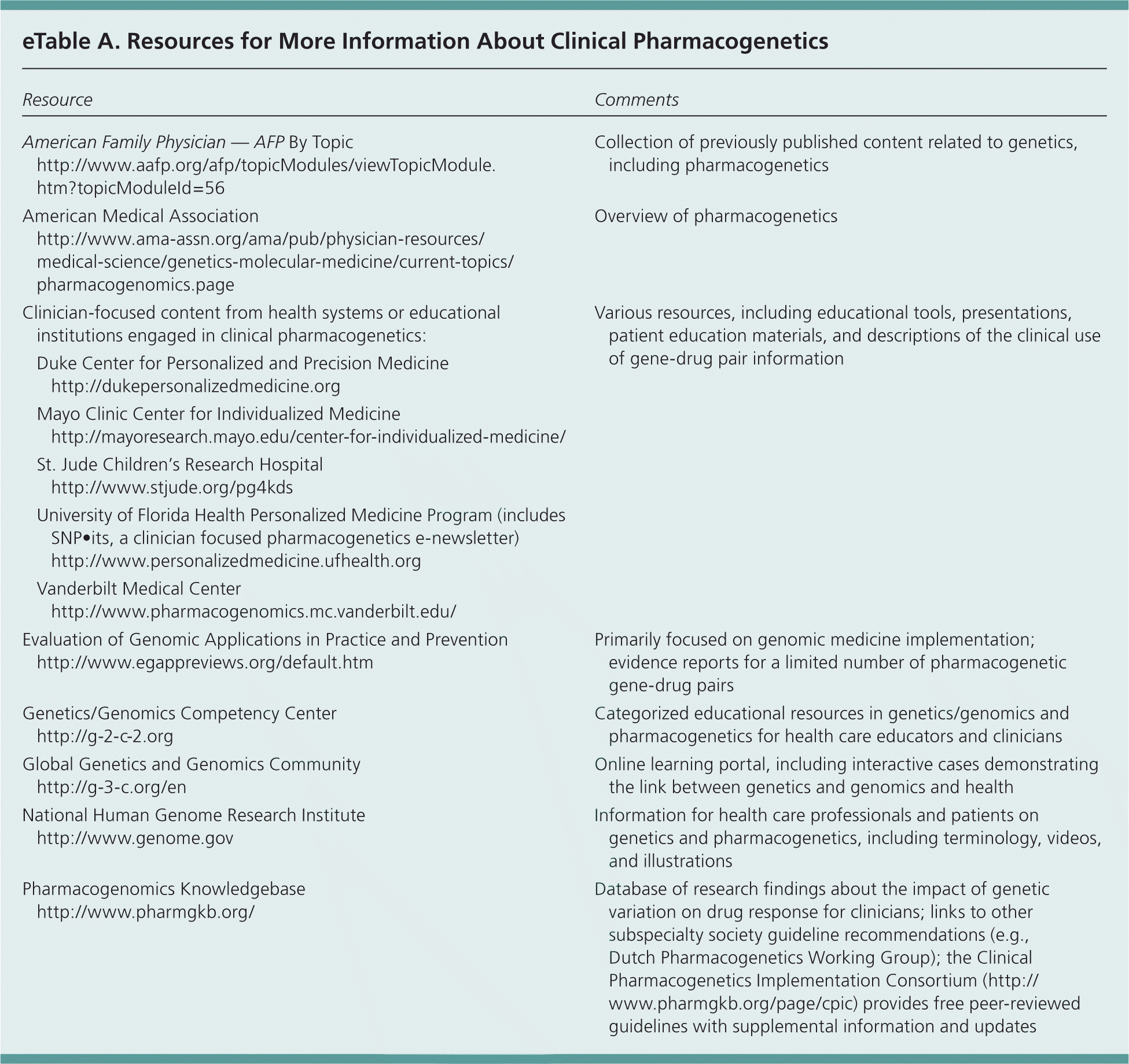
Clinical pharmacogenetics determines whether individual differences in the expression of a protein or enzyme affect the metabolism of a drug. These effects may lead to changes in the levels of active or inactive metabolites, possibly warranting the use of a different drug or dose.1 Family physicians are usually the first resource for patient questions about genetics; however, quick and accurate use of pharmacogenetic data in a clinical environment is challenging.2 Patients have increasing interest in and access to their own genetic information, including pharmacogenetic data from direct-to-consumer genetic testing companies (e.g., 23andMe).3 With pharmacogenetic information on the labels of more than 150 drugs approved by the U.S. Food and Drug Administration (FDA), family physicians should have some knowledge of how to find and apply this information.4 eTable A lists resources for more information.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Codeine should be avoided in CYP2D6 ultrarapid metabolizers because of the potential for toxicity. | C | 13, 23 | Consensus guideline based on observational studies and case reports |
| Codeine, and possibly tramadol, should be avoided in CYP2D6 poor metabolizers because of possible lack of effectiveness. | C | 13, 23 | Consensus guideline based on observational studies |
| In poor CYP2C19 metabolizers who are undergoing percutaneous coronary intervention for acute coronary syndromes, ticagrelor (Brilinta) or prasugrel (Effient) should be considered as an alternative to clopidogrel (Plavix) for antiplatelet therapy. | B | 9, 36, 37 | Consensus guideline based on observational studies; meta-analyses of observational studies show conflicting results |
| Resource | Comments | ||
|---|---|---|---|
| American Family Physician — AFP By Topic | Collection of previously published content related to genetics, including pharmacogenetics | ||
| https://www.aafp.org/afp/topicModules/viewTopicModule.htm?topicModuleId=56 | |||
| American Medical Association | Overview of pharmacogenetics | ||
| http://www.ama-assn.org/ama/pub/physician-resources/medical-science/genetics-molecular-medicine/current-topics/pharmacogenomics.page | |||
| Clinician-focused content from health systems or educational institutions engaged in clinical pharmacogenetics: | Various resources, including educational tools, presentations, patient education materials, and descriptions of the clinical use of gene-drug pair information | ||
| Duke Center for Personalized and Precision Medicine | |||
| http://dukepersonalizedmedicine.org | |||
| Mayo Clinic Center for Individualized Medicine | |||
| http://mayoresearch.mayo.edu/center-for-individualized-medicine/ | |||
| St. Jude Children's Research Hospital | |||
| http://www.stjude.org/pg4kds | |||
| University of Florida Health Personalized Medicine Program (includes SNP•its, a clinician focused pharmacogenetics e-newsletter) | |||
| http://www.personalizedmedicine.ufhealth.org | |||
| Vanderbilt Medical Center | |||
| http://www.pharmacogenomics.mc.vanderbilt.edu/ | |||
| Evaluation of Genomic Applications in Practice and Prevention | Primarily focused on genomic medicine implementation; evidence reports for a limited number of pharmacogenetic gene-drug pairs | ||
| http://www.egappreviews.org/default.htm | |||
| Genetics/Genomics Competency Center | Categorized educational resources in genetics/genomics and pharmacogenetics for health care educators and clinicians | ||
| http://g-2-c-2.org | |||
| Global Genetics and Genomics Community | Online learning portal, including interactive cases demonstrating the link between genetics and genomics and health | ||
| http://g-3-c.org/en | |||
| National Human Genome Research Institute | Information for health care professionals and patients on genetics and pharmacogenetics, including terminology, videos, and illustrations | ||
| http://www.genome.gov | |||
| Pharmacogenomics Knowledgebase | Database of research findings about the impact of genetic variation on drug response for clinicians; links to other subspecialty society guideline recommendations (e.g., Dutch Pharmacogenetics Working Group); the Clinical Pharmacogenetics Implementation Consortium (http://www.pharmgkb.org/page/cpic) provides free peer-reviewed guidelines with supplemental information and updates | ||
| http://www.pharmgkb.org/ | |||
Few primary care physicians are comfortable ordering a pharmacogenetic test or interpreting test results,5,6 often citing a general lack of education in this area.6 This article presents recommendations for two well-studied gene-drug pairs to illustrate the type of information and evidence needed to apply pharmacogenetic data clinically.
Basics of Pharmacogenetic Variability and Terminology
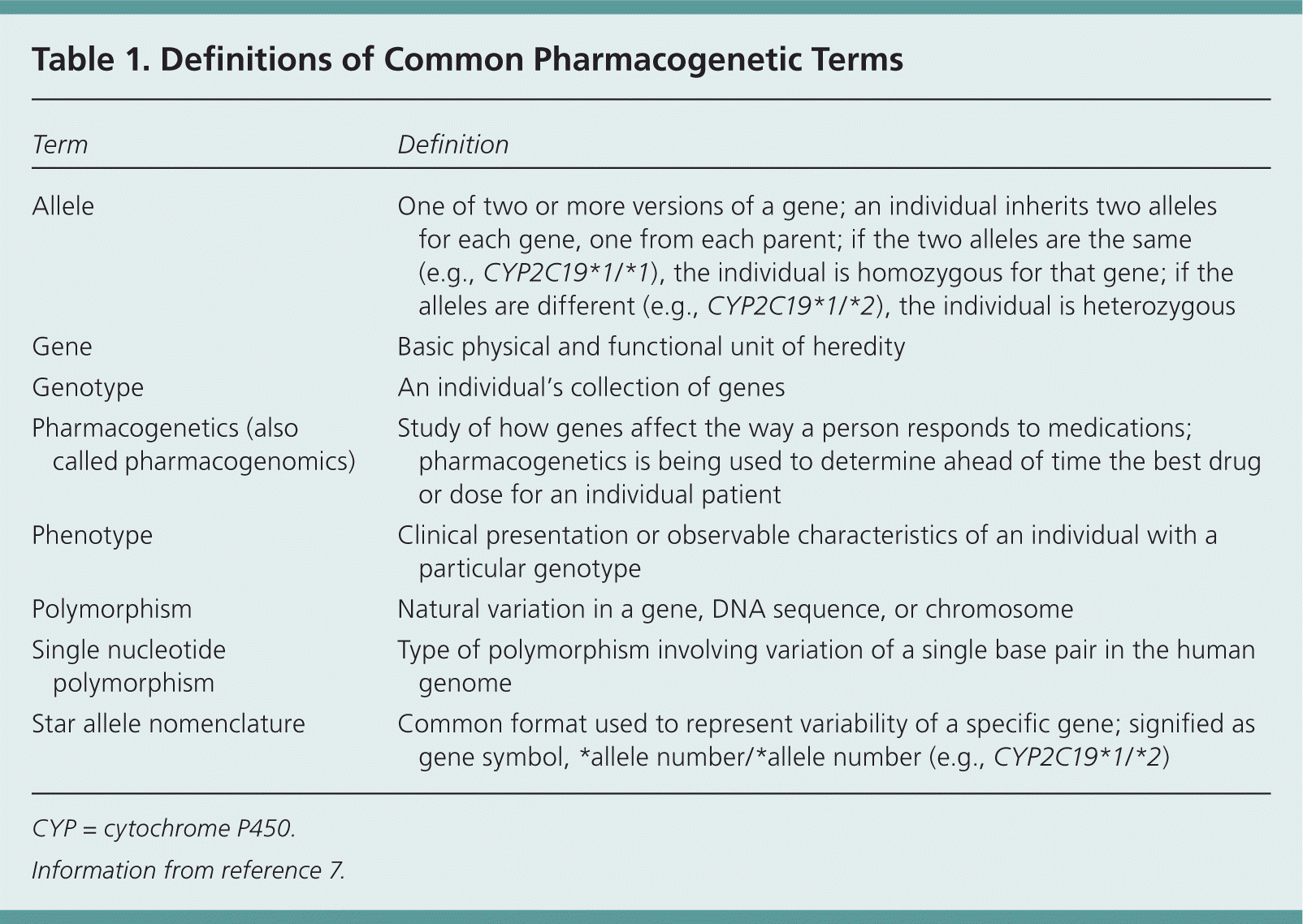
Table 1 includes definitions of commonly used pharmacogenetic terms.7 Much of the available and clinically relevant pharmacogenetic information stems from variations in genes that code for drug metabolizing enzymes (e.g., cytochrome P450 2C19 and clopidogrel [Plavix]), or those that alter a drug's ability to act in the body or the body's response to a drug (e.g., VKORC1 and warfarin [Coumadin]). The most common type of genetic variation (or polymorphism) is a single nucleotide polymorphism. The presence of specific variants at certain single nucleotide polymorphisms or other polymorphisms can lead to different versions of a gene, or alleles. As with many other genetic traits, individuals usually inherit one allele from each parent. These inherited alleles govern expression of the gene and the corresponding enzyme or protein.8
| Term | Definition |
|---|---|
| Allele | One of two or more versions of a gene; an individual inherits two alleles for each gene, one from each parent; if the two alleles are the same (e.g., CYP2C19*1/*1), the individual is homozygous for that gene; if the alleles are different (e.g., CYP2C19*1/*2), the individual is heterozygous |
| Gene | Basic physical and functional unit of heredity |
| Genotype | An individual's collection of genes |
| Pharmacogenetics (also called pharmacogenomics) | Study of how genes affect the way a person responds to medications; pharmacogenetics is being used to determine ahead of time the best drug or dose for an individual patient |
| Phenotype | Clinical presentation or observable characteristics of an individual with a particular genotype |
| Polymorphism | Natural variation in a gene, DNA sequence, or chromosome |
| Single nucleotide polymorphism | Type of polymorphism involving variation of a single base pair in the human genome |
| Star allele nomenclature | Common format used to represent variability of a specific gene; signified as gene symbol, *allele number /*allele number (e.g., CYP2C19*1/*2) |
Pharmacogenetics employs a “star allele” naming system for many genes, in which the normal or reference allele is referred to as wild type and given a designation of *1. A variant allele is usually designated with a * followed by a number other than one to distinguish it from other variants. For example, a patient who carries two wild-type alleles for CYP2C19 would be designated as having a CYP2C19*1/*1 genotype, which is associated with normal CYP2C19 activity (this activity level is the patient's phenotype).8
This genetic variability leads to clinical effects when it changes how drugs are processed or activated in the body. For some genes and drugs, there is evidence to support an association between genetic variability and changes in drug levels or effects. For example, carriage of two reduced-function (or loss-of-function) CYP2C19 alleles, such as CYP2C19*2/*2, is associated with poor metabolization and relatively low CYP2C19 activity. Clopidogrel is a prodrug and requires activation by CYP2C19 to become a bioactive drug. Therefore, patients with this “poor metabolizer” phenotype have reduced active clopidogrel metabolites and higher on-treatment platelet aggregation compared with carriers of CYP2C19*1/*1.8,9
Clinical Implications of Pharmacogenetic Testing
The Clinical Pharmacogenetics Implementation Consortium provides guidance on interpreting genetic test results.10 Additional recommendations are available from the Dutch Pharmacogenetics Working Group and the Evaluation of Genomic Applications in Practice and Prevention working group, and disease-specific guidance is provided in guidelines from various professional associations.11,12
CYP2D6 AND OPIOIDS
Codeine and morphine exert their analgesic effects through interaction at the μ-opioid receptor. The affinity of codeine for this receptor is approximately 200-fold weaker than that of morphine.13,14 As a result, codeine's analgesic properties primarily come from its bioactivation in the liver to morphine via the CYP2D6 enzyme.13,15
CYP2D6 enzyme activity is highly variable because of single nucleotide polymorphisms and other alterations of the CYP2D6 gene.13,14 In approximately 90% of patients, codeine metabolism by CYP2D6 results in expected amounts of morphine formation. However, approximately 1% to 2% of patients are ultrarapid metabolizers, in whom the expected increased formation of morphine leads to a higher toxicity risk. Conversely, the approximately 5% to 10% of patients who are classified as poor metabolizers are at risk of insufficient pain relief with normal codeine dosages.13
A 2006 case report described the death of a breastfed infant of a mother taking codeine.16 The mother was an ultrarapid CYP2D6 metabolizer, and the infant's death was attributed to opioid toxicity secondary to morphine excretion into breast milk. Childhood deaths with normal codeine dosages have been attributed to CYP2D6 polymorphism and resulted in an FDA warning against codeine use for postoperative pain control in children undergoing tonsillectomy or adenoidectomy.17 Adverse effects have also been reported in adults with variant CYP2D6 metabolism.18 FDA-approved prescribing information for codeine warns that even at approved dosages, persons who are ultrarapid metabolizers may have life-threatening or fatal respiratory depression or experience signs of overdose, including extreme sleepiness, confusion, and shallow breathing.19
Other opioids such as tramadol, hydrocodone, and oxycodone are also metabolized through CYP2D6 to their active forms. Of these agents, evidence supporting clinically relevant effects of genetic variability is strongest with use of tramadol in poor metabolizers of CYP2D6. In patients undergoing abdominal surgery, those who were poor metabolizers were more likely to be nonresponsive to tramadol and required significantly more rescue pain medications postoperatively compared with non–poor metabolizers.20,21
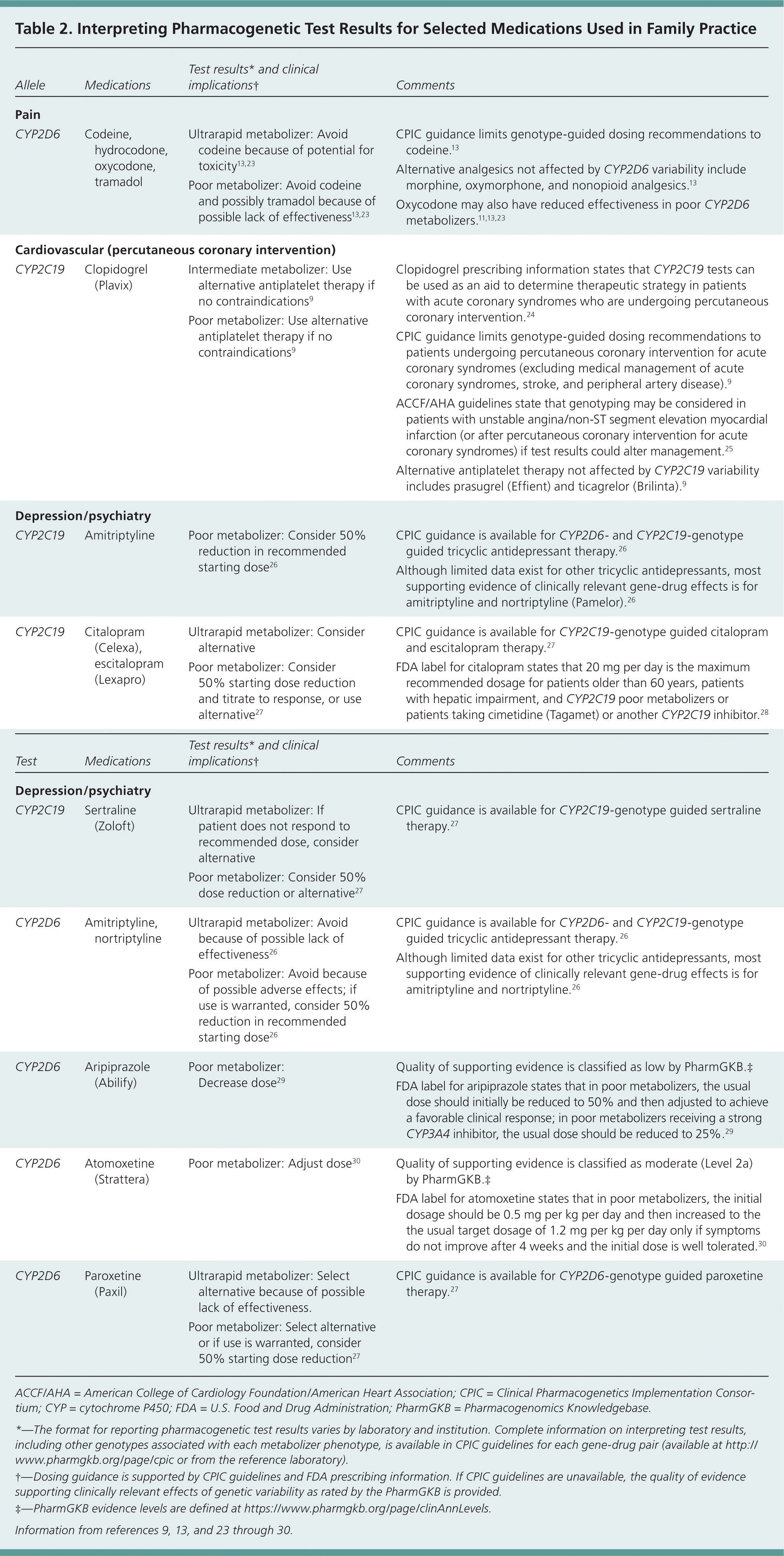
Clinicians should consider CYP2D6 testing in patients who have no response to codeine or tramadol (possible poor metabolizers) or who have unexpected adverse effects (possible ultrarapid metabolizers). Although evidence is limited, clinicians should keep in mind that hydrocodone and oxycodone may not be good alternatives in these patients because they are also activated by this enzyme.13,22 Other factors that may influence optimal drug therapy choices need to be considered, such as concomitant use of drugs that inhibit CYP2D6 (e.g., paroxetine [Paxil]) and risk factors for respiratory depression.13 Numerous other drugs have also been associated with genetic variability in CYP2D6 expression and altered clinical effects (Table 2).9,13,23–30
| Allele | Medications | Test results* and clinical implications† | Comments |
|---|---|---|---|
| Pain | |||
| CYP2D6 | Codeine, hydrocodone, oxycodone, tramadol | ||
| Cardiovascular (percutaneous coronary intervention) | |||
| CYP2C19 | Clopidogrel (Plavix) |
| |
| Depression/psychiatry | |||
| CYP2C19 | Amitriptyline |
| |
| CYP2C19 | Citalopram (Celexa), escitalopram (Lexapro) |
|
|
| CYP2C19 | Sertraline (Zoloft) |
|
|
| CYP2D6 | Amitriptyline, nortriptyline | ||
| CYP2D6 | Aripiprazole (Abilify) |
|
|
| CYP2D6 | Atomoxetine (Strattera) |
|
|
| CYP2D6 | Paroxetine (Paxil) |
|
|
CYP2C19 AND CLOPIDOGREL
Clopidogrel is a prodrug that is activated in the liver to exert its antiplatelet effects by a two-step process that largely involves the CYP2C19 enzyme.31 The CYP2C19 gene is highly polymorphic, with many variations occurring naturally. Most patients (up to 80%) have normal CYP2C19 activity based on their genotype, but approximately 18% to 45% and 2% to 15% of patients have intermediate or poor CYP2C19 enzyme activity, respectively.9,31
Clopidogrel-induced inhibition of platelet aggregation varies widely among different patient groups. This variable response has been linked in part to genetic alterations in the CYP2C19 enzyme function.9 A number of studies have documented decreased formation of active clopidogrel metabolites and higher on-treatment platelet aggregation in patients who carry one or two copies of a reduced-function CYP2C19 allele.31–34
Although clopidogrel has numerous uses, CYP2C19 genetic variability has been linked to adverse clinical outcomes primarily in patients undergoing percutaneous coronary intervention for acute coronary syndromes.9,31,35 Meta-analyses have found that patients undergoing percutaneous coronary intervention for acute coronary syndromes who are poor CYP2C19 metabolizers (carriers of two reduced-function alleles) and taking clopidogrel have a significantly increased risk of a composite outcome of cardiovascular death, myocardial infarction, or stroke (hazard ratio = 1.76; 95% confidence interval, 1.24 to 2.50; P = .002) or stent thrombosis (hazard ratio = 3.97; 95% confidence interval, 1.75 to 9.02; P = .001).9,35,36 Conversely, meta-analyses show no clinical benefit for testing patients with lower clinical risks (e.g., clopidogrel use in atrial fibrillation).9,35,37,38 In 2010, the FDA added a boxed warning to the clopidogrel drug label about higher rates of cardiovascular events in patients undergoing percutaneous coronary intervention for acute coronary syndrome who are poor metabolizers of CYP2C19, compared with patients who have normal CYP2C19 function, and recommended that clinicians consider alternative treatments in these patients.”24
Physicians should consider CYP2C19 testing to guide antiplatelet therapy selection in patients undergoing percutaneous coronary intervention for acute coronary syndromes.9 When evaluating the expected response to clopidogrel, clinicians should keep in mind other factors that may affect clopidogrel response and/or clinical outcomes. These factors include underlying risk factors (e.g., diabetes mellitus, age) and concomitant medications (e.g., omeprazole [Prilosec], a CYP2C19 inhibitor).9,25
Considerations in Ordering and Using Pharmacogenetic Tests
CLINICAL UTILITY AND PRACTICE-BASED RESOURCES
Although a randomized controlled trial is the preferred method for establishing utility of a new drug, there are challenges in designing and executing these trials for pharmacogenetic testing.39–42 Clinical use of pharmacogenetic testing is based largely on observational and retrospective trials conducted in targeted patient populations. Although proponents of pharmacogenetic testing cite challenges in demonstrating the benefit of an intervention that largely benefits patient outliers (e.g., poor metabolizers, nonresponders), the lack of supporting randomized controlled trials remains a limitation in the clinical adoption of these tests.10,40,41,43,44
ORDERING AND REIMBURSEMENT
Although availability varies, CYP2D6, CYP2C19, and other pharmacogenetic tests can be used as stand-alone tests or within broader pharmacogenetic panels. Further information on test availability and ordering is available through the National Institutes of Health Genetic Testing Registry (http://www.ncbi.nlm.nih.gov/gtr/) and the Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/). Because clinical reimbursement rates vary, clinicians should consider contacting the laboratory or the patient's insurance provider for details before ordering.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms pharmacogenetic, pharmacogenomic, CYP2D6, and CYP2C19. The search included clinical trials, randomized controlled trials, systematic reviews, and guidelines. We also searched the National Guideline Clearinghouse database, the Agency for Healthcare Research and Quality evidence reports, DynaMed, and UpToDate. Search dates: September 15, 2014, and June 8, 2015.
This work was supported in part by a grant from the National Institutes of Health, U01 HG007269.
The authors thank Drs. Caitrin McDonough and Larisa Cavallari for their assistance in the preparation of the manuscript.