
Am Fam Physician. 2015;92(8):727-731
Author disclosure: No relevant financial affiliations.
Levomilnacipran (Fetzima) is a serotonin-norepinephrine reuptake inhibitor (SNRI) labeled for the treatment of major depressive disorder in adults.1
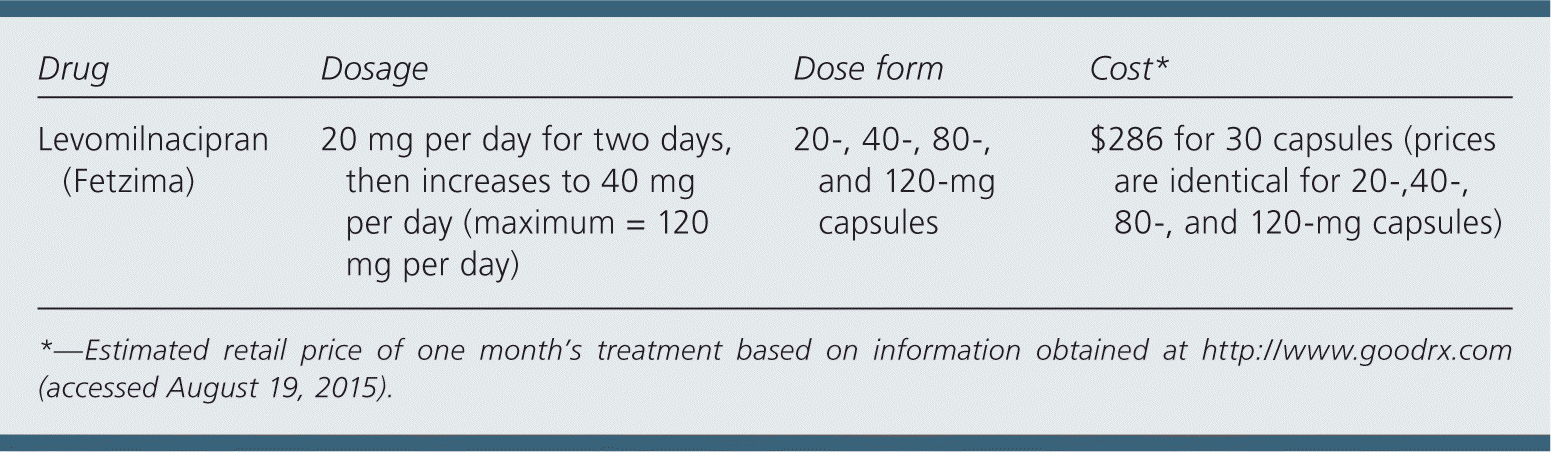
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Levomilnacipran (Fetzima) | 20 mg per day for two days, then increases to 40 mg per day (maximum = 120 mg per day) | 20-, 40-, 80-, and 120-mg capsules | $286 for 30 capsules (prices are identical for 20-,40-, 80-, and 120-mg capsules) |
SAFETY
Levomilnacipran causes few serious adverse effects, although long-term safety has not been established.2 As with all antidepressants, levomilnacipran carries a warning of increased risk of suicidal thoughts and behavior in patients 24 years or younger, and it has not been studied in patients younger than 18 years.1 Levomilnacipran increases systolic and diastolic blood pressure by a median of 4 mm Hg and 2.5 mm Hg, respectively,2 and 3% of patients (95% confidence interval, 1% to 6%) with normal blood pressure or prehypertension will develop hypertension.3 The manufacturer recommends against using it in patients with poorly controlled hypertension.1 Blood pressure should be routinely measured until the effect of levomilnacipran is determined. It does not cause clinically significant changes in basic laboratory tests or QT prolongation.2,4–6 Patients should avoid using levomilnacipran with serotonergic drugs, such as monoamine oxidase inhibitors, triptans, tramadol, and other antidepressants to minimize the risk of serotonin syndrome.1 The dosage of levomilnacipran should be limited to 80 mg per day when used concurrently with a cytochrome P450 3A4 inhibitor because levomilnacipran is partially metabolized by the liver, and medications that inhibit the cytochrome P450 3A4 system, such as ketoconazole, may cause it to accumulate.1 Patients with moderate and severe renal impairment should be limited to a dosage of 80 mg per day and 40 mg per day, respectively. 1
TOLERABILITY
About one in six patients will experience nausea, and about one in 11 patients will experience hyperhydrosis. Other adverse effects include headache, tachycardia, palpitations, constipation, and erectile dysfunction and ejaculation disorder in men.2,4–7 Levomilnacipran does not cause weight gain.2,4,5,7 Adverse effects lead to discontinuation of treatment in approximately 8.8% of patients by eight weeks and 13% of patients by one year.2,3
EFFECTIVENESS
The effect of levomilnacipran on mild to moderate major depression has not been studied. In outpatients with moderate to severe depression, about one in four patients (27.8%) will achieve remission (a score of less than 10 on the Montgomery-Åsberg Depression Rating Scale [MADRS]).3–7 Levomilnacipran induced a treatment response (i.e., more than 50% reduction in the MADRS score) in 44.8% of patients.3–7 Short-term research has not demonstrated an effect of levomilnacipran on relapse rates.8 There is no research comparing levomilnacipran with other antidepressants.
PRICE
A one-month supply of levomilnacipran costs approximately $286. Prices are identical for 20-, 40-, 80-, and 120-mg capsules. In comparison, a 30-day supply of venlafaxine (37.5 mg per day) costs approximately $15, and duloxetine (Cymbalta; 30 mg per day) costs approximately $40.
SIMPLICITY
Levomilnacipran should be taken once daily at the same time of day, with or without food. The starting dosage is 20 mg per day for two days, then increases to 40 mg per day. Based on tolerability and effectiveness, the dosage may be titrated upward by 40 mg every two days to a maximum dosage of 120 mg per day.1 Typical target dosages in flexible-dose clinical studies ranged from 80 mg per day to 120 mg per day.5,6
Bottom Line
Levomilnacipran should not be used in patients with mild to moderate depression until studies have proven its effectiveness. It is somewhat effective in patients with moderate to severe depression. The short-term adverse effect profile of levomilnacipran is similar to that of other SNRIs. However, there is no research comparing it with other antidepressants, and it lacks long-term safety data. Levomilnacipran is also considerably more expensive than other antidepressants.
